the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Plant hydraulic transport controls transpiration sensitivity to soil water stress
Sally E. Thompson
Xue Feng
Plant transpiration downregulation in the presence of soil water stress is a critical mechanism for predicting global water, carbon, and energy cycles. Currently, many terrestrial biosphere models (TBMs) represent this mechanism with an empirical correction function (β) of soil moisture – a convenient approach that can produce large prediction uncertainties. To reduce this uncertainty, TBMs have increasingly incorporated physically based plant hydraulic models (PHMs). However, PHMs introduce additional parameter uncertainty and computational demands. Therefore, understanding why and when PHM and β predictions diverge would usefully inform model selection within TBMs. Here, we use a minimalist PHM to demonstrate that coupling the effects of soil water stress and atmospheric moisture demand leads to a spectrum of transpiration responses controlled by soil–plant hydraulic transport (conductance). Within this transport-limitation spectrum, β emerges as an end-member scenario of PHMs with infinite conductance, completely decoupling the effects of soil water stress and atmospheric moisture demand on transpiration. As a result, PHM and β transpiration predictions diverge most for soil–plant systems with low hydraulic conductance (transport-limited) that experience high variation in atmospheric moisture demand and have moderate soil moisture supply for plants. We test these minimalist model results by using a land surface model at an AmeriFlux site. At this transport-limited site, a PHM downregulation scheme outperforms the β scheme due to its sensitivity to variations in atmospheric moisture demand. Based on this observation, we develop a new “dynamic β” that varies with atmospheric moisture demand – an approach that overcomes existing biases within β schemes and has potential to simplify existing PHM parameterization and implementation.
- Article
(2002 KB) - Full-text XML
-
Supplement
(3095 KB) - BibTeX
- EndNote
Plants control their transpiration (T) and CO2 assimilation by adjusting leaf stomatal apertures in response to environmental variations (Katul et al., 2012; Fatichi et al., 2016). In doing so, they mediate the global water, carbon, and energy cycles. The performance of most terrestrial biosphere models (TBMs) relies on accurately representing leaf stomatal responses in terms of stomatal conductance (gs). Extensive research has established the relationships between gs and atmospheric conditions like photosynthetically active radiation, humidity, CO2 concentration, and air/leaf temperature under well-watered conditions, though the specific forms of these relationships vary (Damour et al., 2010; Buckley and Mott, 2013; Buckley, 2017). However, representing the dynamics of gs in response to soil water stress remains problematic.
Many TBMs represent declining gs and, in turn, transpiration reduction (i.e., downregulation) in response to soil water stress with an empirical function of soil water availability. This method, known as β (Powell et al., 2013; Verhoef and Egea, 2014; Trugman et al., 2018; Paschalis et al., 2020), reduces gs from its peak value under well-watered conditions (gs,ww), i.e., , . (We use the term “β” in this paper to refer to the downregulation model itself, and the terms “β function” and “β factor” to refer to the empirical function and its values, respectively.) The term “well-watered” refers to moist soil conditions where stomatal aperture is unaffected by plant water uptake from the soil, i.e., no soil water stress. The first β-like function appeared, to the best of our knowledge, in an early global heat balance study (Budyko, 1956) to reduce “evaporability” (comparable to well-watered gs and T) for unsaturated land surfaces using a normalized soil moisture value. This method was eventually incorporated into the hydrology component of one of the first global circulation models (Manabe, 1969). However, many current β functions appear to stem from the heuristic root water uptake assumptions originally implemented in the crop transpiration model SWATR (Feddes et al., 1976, 1978), which evolved into the widely used SWAP model (Kroes et al., 2017). Since then, β has gained widespread use within TBMs and hydrological models due to its parsimonious form.
However, mounting evidence indicates that using β in TBMs is a major source of uncertainty and bias in plant-mediated carbon and water flux predictions. Multiple studies have implicated the lack of a universal β formulation as a primary source of inter-model variability in carbon cycle predictions (Medlyn et al., 2016; Rogers et al., 2017; Trugman et al., 2018; Paschalis et al., 2020). For example, different β formulations among nine TBMs accounted for 40 %–80 % of inter-model variability in global gross primary productivity (GPP) predictions (on the order of 3 %–286 % of current global GPP) (Trugman et al., 2018). Aside from the uncertainty in functional form, β appears to fundamentally misrepresent the coupled effects of soil water stress and atmospheric moisture demand on stomatal closure. Recent work using model–data fusion at FLUXNET sites highlighted that β produces stomatal responses that are overly sensitive to soil water stress and unrealistically insensitive to atmospheric moisture demand (Liu et al., 2020). Furthermore, TBM validation experiments have found that β schemes produce unrealistic GPP prediction during drought at Amazon rainforest sites (Powell et al., 2013; Restrepo-Coupe et al., 2017) and systematic overprediction of evaporative drought duration, magnitude, and intensity at several AmeriFlux sites (Ukkola et al., 2016). The apparent inadequacy of β has lead to the adoption of physically based plant hydraulic models (PHMs) in TBMs (Williams et al., 2001; Bonan et al., 2014; Xu et al., 2016; Kennedy et al., 2019; Eller et al., 2020; Sabot et al., 2020).
PHMs represent water transport, driven by a gradient of water potential energy, through the soil–plant–atmosphere continuum via flux-gradient relationships (based on Hagen–Poiseuille flow), which use measurable soil properties and plant traits as parameters (Mencuccini et al., 2019). The implementation of PHMs in several popular TBMs (e.g., CLM, JULES, etc.) has improved predictions in site-specific GPP and evapotranspiration (ET) predictions (Powell et al., 2013; Bonan et al., 2014; Kennedy et al., 2019; Eller et al., 2020; Sabot et al., 2020) as well as soil water dynamics (Kennedy et al., 2019) compared to β. PHMs also exhibit more realistic sensitivity to atmospheric moisture demand than β (Liu et al., 2020). However, these improvements from PHMs come at the cost of an increased number of plant hydraulic trait parameters and computational burden, which can reduce the reliability of the predictions (Prentice et al., 2015). Additionally, obtaining representative plant hydraulic trait values for a soil–plant system is difficult for two main reasons: (i) traits vary widely across and within species (Anderegg, 2015) and exhibit plasticity through acclimation and adaptation (Franks et al., 2014), and (ii) trait measurements are typically made at a single point (e.g., stem, branch, leaf), which may not reliably scale to represent whole-plant or ecosystem-level responses due to the effects of nonlinear trait variations along the soil–plant system (Couvreur et al., 2018). These difficulties result in uncertainty in the model predictions that may be further compounded at the ecosystem level (Fisher et al., 2018; Feng, 2020). Consequently, modelers continue to rely on β as a parsimonious alternative to PHMs (Paschalis et al., 2020).
The relative strengths and weaknesses of β and PHMs suggest that informed model selection requires a better understanding of when the complexity of a PHM is justified over the simplicity of β. This paper informs such understanding by (i) analyzing the fundamental differences between PHMs and β (Sect. 3.1), (ii) defining the parameters controlling the differences (Sect. 3.2), and (iii) demonstrating how PHMs outperform β for a real soil–plant system (Sect. 3.3). Then, leveraging our theoretical insights, we create a new “dynamic β” as a potential tool to correct the biases from the original β while reducing the parameter and computational demands of PHMs (Sect. 3.3). To accomplish these goals, we first analyze a minimalist PHM using a water supply–demand framework, then corroborate the results for a more widely used complex PHM, and, finally, perform a case study with a calibrated land surface model (LSM), which employs β, PHM, and dynamic β downregulation schemes.
2.1 Minimalist PHM
Our minimalist (Sect. 3.1–3.2) and complex PHM formulations (Sect. 3.3), illustrated in Fig. 1, rely on a supply–demand framework that conceptualizes transpiration as the joint outcome of soil water supply and atmospheric moisture demand (Gardner, 1960; Cowan, 1965; Sperry and Love, 2015; Kennedy et al., 2019). In this framework, “supply” refers to the rate of water transport to the leaf mesophyll cells from the soil, into the roots, and through the xylem. “Demand” refers to the rate of water vapor outflux through the stomata, driven by the transport capacity of the air surrounding the plant and regulated by the stomatal response to atmospheric conditions (Buckley, 2017) and leaf water status (Klein, 2014; Buckley, 2019). We assume steady-state transpiration fluxes (i.e., supply equals demand), which means we neglect the effects of plant capacitance (Bohrer et al., 2005) and also assume that the mean plant and atmospheric states equilibrate quickly over short timescales.
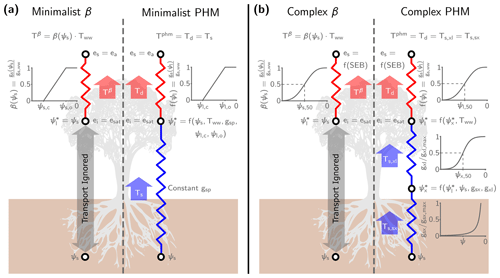
Figure 1Schematic for the minimalist (a) and complex (b) β and PHM models used in this analysis. The resistors represent the conductance between soil–plant segments (i.e., an analogy to Ohm's law) that mediate liquid water supply (blue) and atmospheric water vapor demand (red). Next to each resistor is the segment-specific conductance downregulation curve dependent on water potential (ψ). The white circles indicate segment endpoints where we calculate the potentials (ψ) for liquid water transport and vapor pressures (e) for water vapor transport. The supply segment subscripts represent soil (s), xylem (x), leaf (l), and bulk soil-plant (sp), whereas the demand segment subscripts represent inside the leaf (i), at the leaf surface (s), and the ambient air (a). For water vapor transport, we assume saturation vapor pressure inside the leaf (ei=esat) for both models. In the minimalist models, we assume the leaf surface vapor pressure (es) is the atmospheric vapor pressure (ea), which makes the driving force for water vapor transport the leaf-to-air vapor pressure deficit (). Alternately, in the complex models, es is a function of the surface energy balance (f(SEB)) calculations at each time step. The thick arrows represent the water transport through each segment calculated by the integrated steady-state flux-gradient relationships discussed in Sect. 2.1–2.2 and 2.5. We use the minimalist models (left panel) for Sect. 3.1–3.2 and the complex models (right panel) for the LSM analysis in Sect. 3.3. (Note that we only illustrate a single big-leaf formulation here, but see Sect. S2 for the two-big-leaf implementation.)
The minimalist PHM supply (Ts [mm d−1]; Eq. 1 and blue segment in Fig. 1a) is represented by a steady-state integrated 1-D flux-gradient relationship, bounded by the root-zone-average soil water potential (ψs [MPa]) and leaf water potential (ψl [MPa]) and mediated by the bulk conductance along the flow path (gsp(ψ) ). For simplicity, we assume constant soil–plant conductance (gsp) and ignore its dependence on water potential (i.e., hydraulic limits; Sperry et al., 1998). This assumption simplifies the integral in Eq. (1) to the product of gsp and the water potential difference, ψs−ψl, which drives the flow.
The minimalist PHM demand (Td [mm d−1]; Eq. 2 and red segment in Fig. 1a) uses a similar conductance-difference formulation (i.e., integrated flux-gradient relationship). Transpiration is driven by the leaf-to-air water vapor pressure deficit (D [mol H2O per mol air]) and mediated by the well-watered stomatal conductance (gs,ww ), a stomatal closure term (f(ψl)), and the leaf area index (LAI [m2 leaf m−2 ground]). Additionally, we convert Td from a molar flux to a volume flux using the conversion factor Ca (i.e., the molar weight of water (Mw [kg mol−1]) divided by water density (ρw [kg m−3]) and multiplied by the conversion from m s−1 to mm d−1). The driving force D assumes saturation vapor pressure inside the leaf (i.e., ei=esat) and that the leaf surface (es) and atmospheric vapor pressure (ea) are the same (i.e., the leaf is well-coupled to the atmosphere; Jarvis and McNaughton, 1986); however, the leaf temperature can differ from the atmosphere, which differentiates D from atmospheric vapor pressure deficit (Grossiord et al., 2020). The parameter gs,ww encapsulates the stomatal response to atmospheric conditions only (i.e., light, temperature, humidity, and CO2 concentration). We define the product of LAI, gs,ww, and D as the well-watered transpiration rate (Tww) – which represents atmospheric moisture demand throughout this paper – and we specify its value for the minimalist analysis. The term “well-watered” refers to abundant soil water conditions under which water transport to the leaves maintains ψl high enough to avoid stomatal closure. During water-stressed conditions, the f(ψl) term represents stomatal closure (i.e., downregulating gs,ww) to lowering leaf water status (Buckley, 2019). We assume a normalized, piecewise linear f(ψl) (Eq. 3 and illustrated in Fig. 1a), parametrized by the leaf water potential at incipient (ψl,o) and complete stomatal closure (ψl,c). This simple multiplicative reduction of gs,ww (similar to the approach of Jarvis, 1976) captures the observed non-unique relationship between gs and ψl (Anderegg and Venturas, 2020) while facilitating comparison with the similar minimalist β formulation (see Sect. 2.5).
The PHM supply and demand are coupled through their mutual dependence on leaf water potential. The ψl value that balances supply (Eq. 1) and demand (Eq. 2) – which we will call (Eq. 4) – yields the steady-state transpiration rate for the minimalist PHM (Tphm; Eq. 5). The full derivation of and Tphm is shown in Sect. S1 in the Supplement.
2.2 Complex PHM
The LSM analysis (Sect. 3.3) uses a complex PHM formulation following Feng et al. (2018). The PHM separates supply into soil-to-xylem and xylem-to-leaf segments and demand into a leaf-to-atmosphere segment (Fig. 1b). Here, we briefly discuss the complex PHM components for a single big-leaf formulation; however, we refer the reader to Sects. S2–S3 for full model details and parameter values for the two-big-leaf formulation used in our LSM.
For PHM supply (Ts; blue segments in Fig. 1b), the water potential gradient drives flow through the soil–plant system mediated by the segment-specific conductances. Unlike the minimalist PHM (Sect. 2.1), we assume the conductance in each segment depends on water potential, which represents “hydraulic limits” (Sperry et al., 1998) that arise via (i) the inability of roots to remove water from soil pores at low ψs and (ii) xylem embolism caused by large hydraulic gradients required under low ψs and/or high Tww. The soil-to-xylem conductance (gsx ; Eq. 6 and illustrated in Fig. 1b) is its maximum value (gsx,max) downregulated by the unsaturated soil hydraulic conductivity curve (Clapp and Hornberger, 1978), which is parametrized by the saturated soil water potential (ψsat), soil water retention exponent (b), unsaturated hydraulic conductivity exponent (), and a correction factor (d) to account for roots' ability to reach water (Daly et al., 2004). The xylem-to-leaf conductance (gxl ; Eq. 7 and illustrated in Fig. 1b) is its maximum value (gxl,max) downregulated by a sigmoidal function (Pammenter and Willigen, 1998), which is parametrized by the vulnerability exponent (a) and the xylem water potential (ψx) at 50 % loss of conductance (ψx,50). We estimate the maximum conductance values for each segment (gsx,max and gxl,max) with trait-based equations following Feng et al. (2018) (see Sect. S2.5.3). Given that conductance varies with water potential, we utilize a Kirchhoff transform (Eq. 8) to approximate the water supply from each segment (Ts,sx and Ts,xl [mm d−1]; Eqs. 9–10) as the difference in the matric flux potential (Φ [mm d−1]) at the segment endpoints. Therefore, given a value of ψs (i.e., root-zone-average water potential) and ψl, the ψx that balances Ts,sx and Ts,xl – called – yields the steady-state supply rate (Ts).
The complex PHM demand (Td [mm d−1]; Eq. 11 and red segment in Fig. 1b) mirrors the minimalist version (Eq. 2) with modifications to fit into a dual-source LSM scheme (Sect. 2.3) that explicitly represents the coupled mass, heat and energy transfer between the plant, its microclimate, and the atmosphere. The driving force of transpiration is no longer D (i.e., the leaf-to-air vapor pressure deficit) but rather the difference between leaf internal (ei [kPa]) and surface (es [kPa]) vapor pressure (normalized by atmospheric pressure (Patm [kPa]) to obtain units mol H2O per mol air). We still assume ei is the saturation vapor pressure at leaf temperature (esat), but now es depends on the plant microclimate determined by the LSM energy balance solution at each time step (see Sect. S2.6). This plant microclimate is coupled to the well-watered stomatal conductance (gs,ww ) via the optimality-based stomatal response model of Medlyn et al. (2011). The Medlyn model (Eq. 12) depends on the leaf vapor pressure difference (ei−es [kPa]), net CO2 assimilation rate (An ), and the leaf surface CO2 mole fraction (approximated by the ratio of leaf surface CO2 partial pressure (cs [kPa]) and Patm to give units mol CO2 per mol air) and is parametrized by the minimum stomatal conductance (go ) and a slope parameter (g1 [kPa0.5]). Furthermore, we couple gs,ww to the Farquhar et al. (1980) photosynthesis model through An to ensure CO2 diffusion into the leaf balances carbon assimilation (Collatz et al., 1991) (see Sect. S2.4). As in the minimalist model, the product of gs,ww, driving force, and LAI yields the well-watered transpiration rate, Tww, which we take to represent atmospheric moisture demand. Under water-stressed conditions, we keep a Jarvis-like stomatal closure term (f(ψl)) to downregulate gs,ww, because it facilitates easy comparisons between our minimalist and complex formulations. However, we upgrade f(ψl) from a piecewise linear form (Eq. 3) to a more realistic Weibull form (Eq. 13 and illustrated in Fig. 1b) parametrized by a shape factor describing stomatal sensitivity (bl) and the leaf water potential at 50 % loss of stomatal conductance (ψl,50 [MPa]) (Klein, 2014; Kennedy et al., 2019).
As in the minimalist PHM, the complex PHM supply and demand are coupled through their mutual dependence on ψl. The that balances Ts (found at for Eqs. 9–10) and Td (Eq. 11) yields the steady-state transpiration rate for the complex PHM (Tphm). We numerically calculate this solution by recasting Eqs. (9)–(11) as a nonlinear least squares problem and finding the and that ensure mass balance between the segments (see Sect. S2.5.3).
2.3 LSM description and calibration
We created an LSM to test several transpiration downregulation schemes (Sect. 3.3) and allow for removal of modules (e.g., subsurface heat and mass transfer) that would unnecessarily complicate our comparisons. Our LSM is a dual-source two-big-leaf approximation (Bonan, 2019) adapted from CLM v5 (Oleson et al., 2018) with several key simplifications: (i) steady-state conditions (i.e., no aboveground mass, heat, or energy storage), (ii) neutral atmospheric stability, (iii) implemented the Goudriaan and van Laar (1994) radiative transfer model in lieu of the two-stream approximation (Oleson et al., 2018), and (iv) forced the LSM with soil moisture, soil heat flux, and downwelling radiation data. We refer the reader to Sect. S2 for full model details and justifications. We formulated the LSM in MATLAB and have made our codes available online (Sloan, 2021; https://doi.org/10.5281/zenodo.5129247).
We created separate LSM versions to test five different transpiration downregulation schemes: (i) well-watered (no downregulation), (ii) a single β (βs) with static parameters, (iii) a β separately applied to sunlit and shaded leaf areas (β2L) with static parameters, (iv) a dynamic β with parameters dependent on Tww (βdyn), and (v) a PHM. We calibrated the PHM version using a two-step approach. First, we simulated 13 600 parameter sets using Progressive Latin Hypercube Sampling (Razavi et al., 2019) on 15 soil and plant parameters (Table S6) and selected the best parameter set based on a comparison of RMSE, correlation coefficient, percent bias, and variance to AmeriFlux evapotranspiration, sensible heat flux, gross primary productivity, and net radiation site data (Figs. S5–S8). Unfortunately, the best parameter set contained an unrealistically low ψl,50 value for ponderosa pine compared to observations (DeLucia and Heckathorn, 1989). Therefore, as a second step, we adjusted the ψl,50 and several other soil and plant parameters to more realistic values while ensuring that they replicated the transpiration downregulation behavior of the original parameter set. These parameter adjustments had minimal impact on the LSM predictions as the underlying equations are highly nonlinear, and multiple parameter sets can give near equivalent results (i.e., equifinality). We refer the reader to Sect. S4 for a more detailed account of calibration.
We parametrized the three LSM versions containing the β schemes by calibrating the respective β functions to the relative transpiration outputs () of the calibrated PHM version, while we ran the well-watered version using the calibrated parameters and downregulation turned off. The choice to calibrate a single LSM version ensured that the performance differences between the schemes would be due to the PHM representing plant water use more realistically and not to the artifact of differing parameter fits between LSM versions. We refer the reader to Sect. S6.2 for specific details of the parameter fits for the β schemes.
2.4 Site description and forcing data
We calibrated and forced the LSM with half-hourly data from the US-Me2 “Metolius” AmeriFlux site (Irvine et al., 2008) for daylight hours during May-August 2013-2014. The forcing data were taken from both the AmeriFlux (Law, 2021) and FLUXNET2015 (FLUXNET2015, 2019; Pastorello et al., 2020) data products (see Sect. S5 for full details). The site consists of intermediate-age ponderosa pine trees on sandy loam soil in the Metolius River basin in Oregon, USA. We selected this site specifically for its subsurface soil moisture and temperature profiles as well as its separate measurements of photosynthetically active radiation (PAR) and near-infrared radiation (NIR). We used these boundary condition data to force the LSM in lieu of solving one-dimensional subsurface mass and heat transfer equations and atmospheric radiation partitioning models. In particular, we forced the LSM with root-zone-averaged soil water potential (ψs; estimated from measured soil water content and a pedotransfer function) and the ground heat flux measurements. We selected the measurement depth of 50 cm to represent ψs based on the deviation of measured GPP from its mean in relation to measured soil water content and vapor pressure deficit (Fig. S10). The 50 cm measurements showed clear GPP downregulation under water stress. Furthermore, the depth seemed reasonable given previous modeling at this site estimated an effective rooting depth of 1.1 m (Schwarz et al., 2004). The atmospheric forcing for the LSM consisted of incoming direct and diffuse NIR and PAR fluxes, CO2 concentration, atmospheric pressure, vapor pressure, temperature, and wind velocity at the measurement tower height of 32 m. Full description of the forcing data is given in Sect. S5.
2.5 β formulations
The β function empirically represents stomatal closure to declining leaf water status caused by soil water stress. By design, β makes the simplifying assumption that stomata respond directly to soil water status (to avoid the complexity of implementing a PHM as illustrated by Fig. 1), which is readily available in TBM subsurface hydrology schemes as ψs or volumetric soil water content (θs). This heuristic approach leads to multiple β functions based on modeler preference (see the supplement of Trugman et al., 2018, for a list of differing β formulations common to TBMs). Furthermore, even if a universal β function existed, there is open debate on how to apply the β factor (Egea et al., 2011); some TBMs apply the β factor directly to stomatal conductance (Kowalczyk et al., 2006; De Kauwe et al., 2015; Wolf et al., 2016), whereas others indirectly affect stomatal conductance by applying the β factor to photosynthetic parameters (Zhou et al., 2013; Lin et al., 2018; Kennedy et al., 2019). Here, we select a single β formulation that easily compares with the demand component of our PHM. Selecting a different β formulation could alter our values; however, we do not expect our main conclusions about β and PHM differences to change as long as two criteria are met. First, the stomatal downregulation factors for the PHM (f(ψl)) and β (β(ψs)) are applied consistently in the transpiration downregulation scheme (to either gs,ww or photosynthetic parameters). Second, if β is in terms of θs, a curvilinear form must be used (Egea et al., 2011) to ensure β can be mapped approximately to the water potential space of our analysis.
In this paper, we have defined the β function in terms of ψs and apply the β factor directly to gs,ww and, in turn, Tww (Eq. 14) for three key reasons: (i) water transport through the soil–plant–atmosphere continuum follows a gradient of water potential, not water content, (ii) β using ψs rather than θs produces more realistic downregulation behavior compared to data (Verhoef and Egea, 2014), and (iii) applying the β factor to gs,ww directly corresponds to the PHM demand in both minimalist and complex formulations. In the minimalist analysis (Sect. 3.1–3.2), β(ψs) (Eq. 15 and illustrated in Fig. 1a) takes a piecewise linear form (analogous to Eq. 3), which is parametrized by the soil water potential at incipient (ψs,o) and complete stomatal closure (ψs,c). Similarly, in the LSM analysis (Sect. 3.3), β(ψs) (Eq. 16 and illustrated in Fig. 1b) takes a Weibull form (analogous to Eq. 13) parametrized by the soil water potential at 50 % loss of stomatal conductance (ψs,50) and a stomatal sensitivity parameter (bs). The LSM analysis uses two versions of Eq. 16: (i) a static version with constant bs and ψs,50 (used by the βs and β2L schemes) and (ii) a dynamic version where bs and ψs,50 are linear functions of Tww (used by the βdyn scheme). We refer the reader to Fig. S12 for illustrations of the different β versions.
3.1 β as a limiting case of PHMs with infinite conductance
The supply–demand framework reveals that the minimalist PHM and β fundamentally differ in their coupling of the effects of soil water stress (represented by ψs) and atmospheric moisture demand (represented by Tww) on transpiration. The PHM supply lines (red lines in Fig. 2a) illustrate soil-to-leaf water transport (Eq. 1) at a fixed soil water availability (ψs) under increasing pull from the leaf (lower ψl) and constant soil–plant conductance (gsp; supply line slope). The PHM demand lines (black lines in Fig. 2a) illustrate transpiration reduction under lower ψl (from stomatal closure) for two Tww values. The supply and demand lines intersect at the minimalist PHM solution ( and Tphm; Eqs. 4–5). Therefore, the minimalist PHM couples the effects of soil water stress to atmospheric moisture demand on transpiration downregulation, because leaf water potential responds to ψs and Tww until it reaches the point of steady-state transpiration (i.e., ).
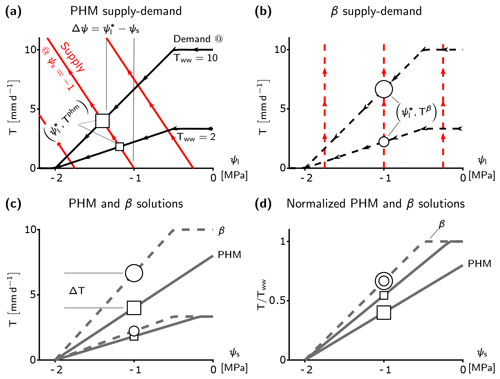
Figure 2Fundamental differences between minimalist PHM and β. (a–b), Supply (red) and demand (black) curves for PHM (a, solid lines) and β (b, dashed lines) under varying leaf water potentials (ψl). The squares (circles) represent the PHM (β) solution – i.e., the where supply equals demand – for a single soil water availability (ψs) and two atmospheric moisture demands (Tww). These markers carry through panels (c) and (d) to illustrate how the solutions between the PHM and β diverge at a single ψs. The relative size of the markers indicates corresponding Tww. The water potential difference Δψ required to transport water from soil to leaf is shown in panel (a) for MPa and Tww=10 mm d−1. (c) Solutions of panels (a) and (b) mapped to ψs, where ΔT is the difference between PHM and β transpiration estimates at MPa and Tww=10 mm d−1. (d) Relative transpiration, in which solutions in panel (c) are normalized by Tww. The β solutions collapse to a single curve, whereas the PHM solutions depend on Tww.
The minimalist β transpiration rate (Tβ; Eq. 14) ignores this coupling as the β function depends only on ψs and independently reduces Tww (shown in Fig. 1). The conditions leading to the decoupling in β only arise if the supply lines are vertical (Fig. 2b), which results in the relative transpiration () depending on ψs only (single curve in Fig. 2d). Since gsp is the supply line slope (Eq. 1), β represents a limiting case of the PHM in which the soil–plant system is infinitely conductive. More specifically, as gsp increases, the leaf water potential approaches the soil water potential (; Eq. 17) and the PHM transpiration rate approaches the β transpiration rate (Tphm→Tβ; Eq. 18). Therefore, the β(ψs) function (Eq. 15) equals the f(ψl) function (Eq. 3) in PHMs and represents stomatal closure to declining leaf (or soil) water potential. In summary, the empirical β physically represents an infinitely conductive soil–plant system where stomata close in response to leaf water potential that depends solely on the soil water potential with which it is equilibrated.
The PHM coupling results in greater transpiration downregulation compared to β under the same environmental conditions (Fig. 2c). For a given soil water stress (ψs), β assumes and downregulates any atmospheric moisture demand (Tww) value by a fixed proportion (i.e., it scales linearly with Tww); hence, it can be modeled with a single curve (Fig. 2d). Conversely, the PHM (with finite conductance) requires a water potential difference () to transport water from soil to leaf; therefore, must be less than ψs, and greater stomatal closure results (Fig. 2c). Furthermore, the PHM downregulates transpiration at a greater proportion with increasing Tww (i.e., it scales nonlinearly with Tww) as it requires a greater Δψ and lower (Fig. 2d). Hence, PHMs require transpiration downregulation to be described as a function of both ψs and Tww.
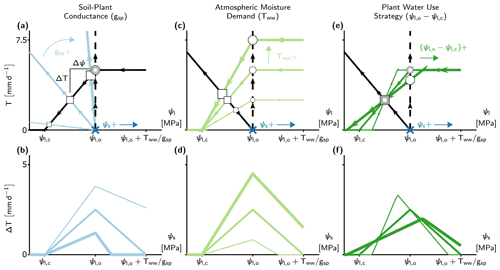
Figure 3The effect of soil water potential (ψs), soil–plant conductance (gsp), atmospheric moisture demand (Tww), and plant water use strategy () on differences between the minimalist PHM and β models (ΔT). (a, c, e) Supply–demand curves at a single soil water availability (indicated by the dark blue x at ) for three prescribed values of gsp, Tww, and , respectively. Each parameter (gsp, Tww, or ) is set at 50 % above (below) its base values at , , , and using thick (thin) colored lines. The squares (circles) indicate the PHM (β) solutions, with size corresponding to magnitude of the changing parameter values. Note that the vertical distance between a correspondingly sized circle and square is ΔT, and horizontal distance is Δψ. (b, d, f) The ΔT results from panels (a), (c), and (e) calculated for a range of ψs with line thickness proportional to parameters in the aforementioned panels (e.g., thick blue line in panel b corresponds to 50 % increase in gsp shown in panel a). The x axes are mapped from ψl in the top panels to ψs in the bottom panels.
These minimalist model results suggest that the range of soil–plant conductances (gsp) can generate a spectrum of possible transpiration responses to soil water stress (and atmospheric moisture demand). Two classes of behaviors emerge – one in a “soil-limited” soil–plant system, in which gsp is large enough for ψl≈ψs, thus decoupling the effects of soil water stress and atmospheric moisture demand while allowing the relative transpiration to vary only with ψs (Fig. 2d). The other class of behavior arises in “transport-limited” systems with finite gsp, in which a non-negligible water potential difference (Δψ) is required to transport the water to the leaf, resulting in additional downregulation compared to soil-limited systems (Fig. 2c) and requiring relative transpiration to depend on both ψs and Tww (Fig. 2d).
3.2 Parameters controlling the divergence of β and PHMs
The differences in PHM and β transpiration estimates (ΔT) depend not only on gsp but also on soil water availability (ψs), atmospheric moisture demand (Tww), and plant water use strategy (). To disentangle these joint dependencies, we adjust a single variable and explore the impact on ΔT using the supply and demand lines (Fig. 3). The translation of supply lines represents ψs changes (indicated in Fig. 3a, c, e) and produces a non-monotonic relationship with ΔT over the range of soil water stress (i.e., ) (Fig. 3b, d, f). The peak ΔT occurs at the incipient point of stomatal closure (ψl,o) as (i) when , transpiration begins to decrease, and in its extreme limit, transpiration (and thus ΔT) approaches 0, and (ii) when , the effects of downregulation diminish in both models as the soil becomes well-watered. The ΔT–ψs behavior acts as a baseline relationship in the following analysis of gsp, Tww, and controls.
The ΔT–ψs relationship increases with lower gsp (Fig. 3b; greater transport limitation) because flatter supply lines increase Δψ (Fig. 3a), requiring greater stomatal closure and hence additional downregulation for a PHM compared to β. Similarly, higher Tww increases the ΔT–ψs relationship (Fig. 3d), although the increase in Δψ stems from steeper demand line slope (Fig. 3c). In addition to increasing ΔT at each ψs value, the effects of gsp and Tww increase the range of soil water stress above ψl,o (up to saturated soil water potential). This result indicates that PHMs can simulate transpiration downregulation under moist soil conditions that β potentially misses as it does not account for large Δψ values from transport limitation and/or high atmospheric moisture demand. Finally, as gsp increases (soil-limited) and Tww decreases, ΔT tends to zero, once again, for slightly different reasons: for gsp, the supply lines approach the β assumption (vertical dashed line in Fig. 3a), whereas for Tww, transpiration approaches zero.
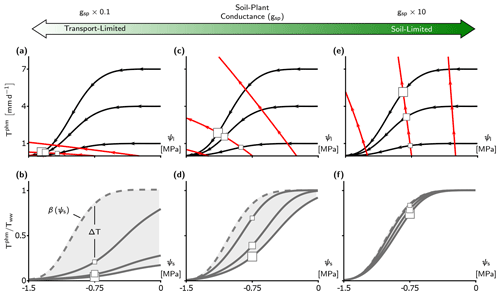
Figure 4Transport-limitation spectrum observed in the complex PHM formulation. (a, c, e) Supply–demand curves for three values of soil–plant conductance, gsp, using the complex PHM formulation. Panel (c) uses the calibrated LSM parameters from the US-Me2 AmeriFlux site discussed in Sect. 2.3. Panels (a) and (e) contain the calibrated conductance () multiplied by 0.1 and 10, respectively. The supply lines (red) are shown at ψs equal to 0, −0.75, and −1.5 MPa, and demand lines (black) are shown at Tww equal to 1, 4, and 7 mm d−1. The PHM solution for ψs at −0.75 MPa is shown by the squares with size corresponding to Tww magnitude. (b, d, f) The relative transpiration for the PHM (solid) in panels (a), (c), and (e) and the infinitely conductive β solution (dashed line) are shown. The light gray shading indicates the PHM downregulation envelope bounded by β(ψs) as Tww approaches zero and the relative transpiration curve at the highest Tww.
Lastly, we explore the effect of plant water use strategy () on ΔT – which approximates the sensitivity of stomatal closure to ψl. Altering does not affect Δψ like the other three variables; however, it modifies the range of soil water stress and redistributes ΔT to conserve the total error over the range. For example, a more aggressive plant water use strategy – closing stomata over a narrower range of ψl and ψs – creates a narrower range of soil water stress with a more peaked ΔT–ψs relationship due to more vertical demand lines (Fig. 3e). Therefore, whether the plant water use strategy could amplify or diminish ΔT for a soil–plant system relies on how site-specific soil moisture variability overlaps with the range of soil water stress (Fig. 3f).
In summary, this minimalist analysis suggests that PHMs are most needed to represent transport-limited soil–plant systems under high atmospheric moisture demand and moderate soil water availability. Plant water use will modulate these results; however, the impact depends on how site-specific soil moisture variability overlaps with the range of soil water stress.
3.3 Improving transpiration predictions with a PHM and a dynamic β
We now perform a modeling case study of the AmeriFlux US-Me2 ponderosa pine site (Sect. 2.4) using our own calibrated LSM (Sect. 2.3) with five separate transpiration downregulation schemes: (i) well-watered (no downregulation), (ii) single β (βs), (iii) β separately applied to sunlit and shaded leaf areas (β2L), (iv) βdyn, and (v) PHM. Specifically, we aim to (i) verify the transport-limitation spectrum from the minimalist analysis (Sect. 3.1) for a complex PHM formulation common to TBMs, (ii) identify errors incurred by selecting β over a PHM (Sect. 3.2) for a real transport-limited soil–plant system, and (iii) develop a new dynamic β that approximates a PHM with simple modifications to the existing β.
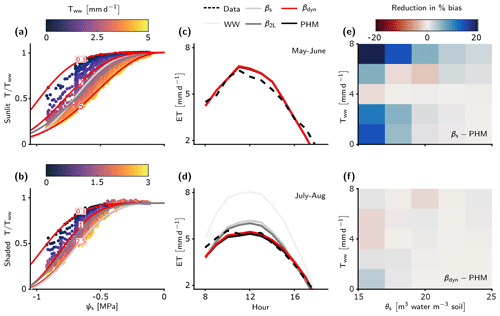
Figure 5LSM evapotranspiration estimates improved by PHM and new dynamic β. (a–b) Fits of the βs, β2L, and βdyn schemes to the relative transpiration outputs from the calibrated PHM scheme for the sunlit (a) and shaded big leaf (b) of the LSM (see Methods section). Note that only three of the infinite family of βdyn curves are shown for illustration – each corresponding to a fixed Tww value in mm d−1 (red numbers). Full fitting details of these three schemes are available in Sect. S6.2. (c–d) The median diurnal ET estimates for the LSM with five transpiration downregulation schemes compared to observations at the US-Me2 AmeriFlux site for early (c) and late summer (d). The dual-source two-big-leaf LSM calculates ET as the sum of sunlit and shaded big-leaf transpiration and ground evaporation. Note that βdyn (red) is overlying PHM (black) results as they are essentially the same. (e–f) Reduction in absolute percent bias of ET between the βs and PHM schemes (e) and βdyn and PHM schemes (f) in terms of atmospheric moisture demand (represented by Tww) and soil water status (represented by θs). In both plots, blue indicates PHM improvement over the selected β scheme.
To aid our comparison of LSM transpiration downregulation schemes, we must first verify that the spectrum of transport limitation found in our minimalist analysis (Sect. 3.1) adequately describes the differences between PHM and β formulations common to TBMs. Our calibrated LSM uses a complex PHM formulation (Sect. 2.2 and Fig. 1b) that partitions the soil–plant–atmosphere continuum into soil-to-xylem, xylem-to-leaf, and leaf-to-atmosphere segments, each with conductance curves that depend nonlinearly (e.g., sigmoidal or Weibull) on water potential. This added complexity does not affect the spectrum of transport limitation (Fig. 4). For clarity, we reiterate two main points from the minimalist PHM analysis found in this complex analysis. First, soil–plant conductance (gsp) controls whether the soil–plant system is soil-limited (high gsp; Fig. 4e–f) or transport-limited (low gsp; Fig. 4a–b) due to non-negligible water potential differences (Δψ) creating large differences between PHMs and β (high ΔT) at intermediate ψs values (Fig. 4b, d). Second, for a transport-limited system, ΔT increases with higher variability in atmospheric moisture demand (Tww), where the importance of “variability” expands on our minimalist results. To clarify, β should be considered an empirical model that could be fit anywhere within the range of the PHM downregulation envelope (light gray shading in Fig. 4b, d, f). Therefore, greater Tww variability creates a larger PHM downregulation envelope and makes a single β increasingly inadequate for modeling transpiration downregulation.
The consistency between the minimalist and complex PHM suggests that the divergence between PHMs and β in transport-limited systems is not sensitive to the linear or nonlinear forms of supply or demand lines but is rather controlled by the existence of a finite conductance itself. Furthermore, these results strongly support the need to use two independent variables, ψs and Tww (rather than only ψs in β), to capture the coupled effects of soil water stress and atmospheric moisture demand on transpiration downregulation in transport-limited soil–plant systems. In light of these findings, we have developed a new dynamic β (βdyn) that has an additional functional dependence on Tww (Eq. 16) and compared it against four other downregulation schemes in this LSM analysis.
We now assess the errors incurred by using a β rather than PHM downregulation scheme to model the US-Me2 ponderosa pine site. The median diurnal evapotranspiration (ET; bare soil evaporation plus transpiration) for each LSM version for early summer 2013–2014 indicates that all downregulation schemes perform similarly due to high soil moisture and minimal downregulation (Fig. 5c). However, as soil moisture declines during late summer (Fig. S11) the differences between schemes emerge: the PHM and βdyn schemes fit the ET observations the best, while β2L, βs, and well-watered schemes overpredict ET (Fig. 5d). We explain the poor performance of the static β schemes by plotting the reduction in absolute percent bias between the βs and PHM schemes (Fig. 5e) with respect to soil water stress (represented by volumetric soil water content measurements at the site, θs [m3 water per m3 soil]) and atmospheric moisture demand (represented by Tww from the well-watered LSM version). The PHM scheme provides substantial percent bias reduction relative to the static βs scheme under soil water stress (θs<0.2) for above- and below-average Tww values (). This result is true for both static β schemes (βs and β2L), because they are fit to the average Tww behavior over the simulation period (Fig. 5a–b and Sect. S6.2). Therefore, as Tww becomes higher (lower) than the average, these static β schemes will overpredict (underpredict) transpiration. The PHM also improves performance during wetter soil conditions (θs>0.2) with high Tww – which does not represent typical “drought” conditions – suggesting that PHMs capture transpiration downregulation that β potentially misses as it cannot account for large soil–plant water potential differences (Δψ) under transport limitation and/or high atmospheric moisture demand (similar to Sect. 3.2). Lastly, the near-average Tww conditions lead to β providing enhanced performance, which can be explained by underlying biases in the calibrated parameter estimates (see Fig. S9).
Notably, the βdyn downregulation scheme replicates the performance of the PHM scheme by adding a single dimension of Tww to the original β scheme. This additional dependence on Tww allows βdyn to traverse along the PHM downregulation envelope with atmospheric moisture demand changes, whereas the static β schemes are fixed near mean conditions (Fig. 5a–b). The performance difference between PHM and βdyn schemes is minimal in terms of percent change in bias across all environmental conditions (Fig. 5f; max difference of 3 %), median diurnal variations (Fig. 5c–d), and cumulative flux errors (Table S7–S8; max difference of 0.5 %). Therefore, this additional dependence on Tww is key to simulating the coupled effects of atmospheric moisture demand and soil water stress in PHMs and accurately modeling transpiration downregulation in transport-limited systems. For this transport-limited system, βdyn requires two more parameters than the original β scheme, which is half the parameters required for our complex PHM formulation (Sect. S6.2). Furthermore, βdyn does not require the iterative solution of water potentials and transpiration in PHMs (Sect. 2.2). Rather, it calculates transpiration downregulation algebraically using ψs as in the original β. The βdyn provides a future avenue for correcting existing β model bias without adding the computational and parametric challenges of more realistic PHMs.
The spectrum of transport- and soil-limited transpiration (Fig. 4) explains why many TBMs that use β to represent transpiration downregulation struggle to predict water, energy, and carbon fluxes under soil water stress (Sitch et al., 2008; Powell et al., 2013; Medlyn et al., 2016; Ukkola et al., 2016; Restrepo-Coupe et al., 2017; Trugman et al., 2018) and why implementing PHMs has led to performance improvements (Kennedy et al., 2019; Anderegg and Venturas, 2020; Eller et al., 2020; Sabot et al., 2020). Transpiration in a transport-limited soil–plant system, characterized by finite soil–plant conductance, depends on non-negligible water potential differences to transport water from the soil to the leaf, which result from the joint effects of atmospheric moisture demand and soil water supply on leaf water potential. It is only when the soil–plant conductance becomes infinite (and the system becomes soil-limited) that leaf water potential approximates soil water potential, and transpiration arises as an independent function of soil water supply and atmospheric moisture demand. These are assumptions inherent to the empirical β and explain why β cannot capture the coupled effects of soil water stress and atmospheric moisture demand.
The implications of continued use of β will vary by site. Ecosystems with soil or plant hydraulic properties resistant to flow (e.g., xeric ecosystems, tall trees, species with low xylem conductivity or roots that hydraulically disconnect from the soil during drought) will have large biases depending on the range of soil water availability and atmospheric moisture demand (Tww) observed at the site (Figs. 3d and 4b). These errors will not be confined to drought periods, as higher atmospheric moisture demand and lower soil–plant conductance can result in errors even during wetter soil conditions (Figs. 3 and 5e). This is a crucial point, given projections indicate diverging degrees of vapor pressure deficit (VPD) stress and soil water stress for ecosystems (Novick et al., 2016). On the other hand, for soil-limited systems (e.g., irrigated crops, riparian vegetation, or groundwater-dependent ecosystems), β may adequately capture transpiration dynamics as soil water status may be a suitable proxy for leaf water status. Therefore, further work must identify the combinations of soil parameters and plant hydraulic traits that define transport- or soil-limited systems to identify ecosystems susceptible to bias from β. Our initial estimates indicate a soil–plant conductance value around 30 may be a rough threshold for transport limitation (see Sect. S7).
Several other factors not covered in this work could exacerbate the differences between β and PHM predictions. We expect plant capacitance (already incorporated into some TBMs; Xu et al., 2016; Christoffersen et al., 2016) will likely cause further deviations from β. PHMs with capacitance are expected to introduce hysteresis into transpiration downregulation (Zhang et al., 2014) in transport-limited systems that existing β are not equipped to capture. However, this hysteretic behavior may diminish in a high-conductance (i.e., soil-limited) system, because plant and soil water potentials will quickly equilibrate, so β may still be an adequate alternative to a PHM. More advanced representation of stomatal response and plant hydraulic transport could further exacerbate β and PHM differences. Recent advances in optimality-based (Eller et al., 2020; Sabot et al., 2020) and mechanistic stomatal response models (Buckley, 2017) as well as more detailed PHM segmentation (Kennedy et al., 2019) may include additional couplings to plant water and metabolism that cannot be easily approximated by β. Regardless, the core message of this work is still relevant: for transport-limited soil–plant systems, PHMs are necessary to couple the effects of soil water stress and atmospheric moisture demand on transpiration, and β fails because soil water status is not an adequate substitute for leaf water status.
The recognition that a dynamic β model can replicate the complexity of a PHM with half the parameters and more direct computation (Sect. S6.2), simply by adding a dependence on atmospheric moisture demand to the β function, provides a useful pathway for overcoming both the limitations of β and the parametric uncertainties of PHMs (Anderegg and Venturas, 2020; Paschalis et al., 2020). The inadequacies of the static β have been noted since its inception. Feddes et al. (1978), who introduced one of the first β formulations, mentioned β's dependence on atmospheric moisture demand based on field data (Denmead and Shaw, 1962; Yang and de Jong, 1972) and early plant hydraulic theory (Gardner, 1960). Unfortunately, there have been only a few attempts to rectify these inadequacies in the modeling community, short of implementing a full PHM. For example, Feddes and Raats (2004) updated their original β model to vary the water potential at incipient stomatal closure linearly with atmospheric moisture demand, which has been adopted in the field-scale SWAP model (Kroes et al., 2017), while the Ecosystem Demography-2 model (Medvigy et al., 2009) uses a sigmoidal function for transpiration downregulation that contains the ratio of soil water supply to evaporative demand. Within many TBMs and hydrological models, a dynamic β could easily replace the original β by allowing existing fixed parameters to vary with Tww (already calculated in many transpiration downregulation schemes). In addition to improving TBM performances, dynamic β also has the potential to aid in remote sensing retrievals and indirect inferences of land surface fluxes. Currently, the state-of-the-art ECOSTRESS (ECOsystem Spaceborne Thermal Radiometer Experiment on Space Station) experiment provides global ET estimates based on a modified Priestley–Taylor formulation that uses a β function to downregulate ET under soil water stress (Fisher et al., 2020). These spaceborne products could easily implement the dynamic β formulation to correct biases for many transport-limited ecosystems. These potential applications rely on formalizing the relationship between the dynamic β parameters and their dependence on Tww. As it stands, the dynamic β still needs to be calibrated to site-specific data; however, it provides a physically informed alternative to PHMs with less calculation and fewer parameters. Further work will focus on generalizing the dynamic β by linking its parameters to measurable soil properties, plant hydraulic traits, and atmospheric feedbacks.
Our custom MATLAB codes for the land surface model used in this paper are freely available at https://doi.org/10.5281/zenodo.5129247 (Sloan, 2021).
The flux data from the US-Me2 ponderosa pine site used in this analysis were downloaded from two publicly available sources. The environmental forcings and measured surface fluxes at US-Me2 were taken from the FLUXNET2015 data product (Pastorello et al., 2020) available at https://fluxnet.org/data/fluxnet2015-dataset/ (last access: 2 January 2019) (FLUXNET2015, 2019). The soil moisture measurements were taken from the AmeriFlux data product (Law, 2021) available at https://doi.org/10.17190/AMF/1246076.
The supplement related to this article is available online at: https://doi.org/10.5194/hess-25-4259-2021-supplement.
XF and SET conceived the idea. BPS and XF designed the research. BPS performed the research. BPS and XF wrote the paper. And ST contributed to refining results and revising the paper.
The authors declare that they have no conflict of interest.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Brandon P. Sloan and Xue Feng acknowledge support from National Science Foundation award DEB-2045610. Brandon P. Sloan and Xue Feng also acknowledge the resources from The Minnesota Supercomputing Institute used to run the simulations in this work.
This paper was edited by Marie-Claire ten Veldhuis and reviewed by Stefano Manzoni and two anonymous referees.
Anderegg, W. R. L.: Minireview Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation, New Phytol., 205, 1008–1014, https://doi.org/10.1111/nph.12907, 2015. a
Anderegg, W. R. L. and Venturas, M. D.: Plant hydraulics play a critical role in Earth system fluxes, New Phytol., 226, 1535–1538, https://doi.org/10.1111/nph.16548, 2020. a, b, c
Bohrer, G., Mourad, H., Laursen, T. A., Drewry, D., Avissar, R., Poggi, D., Oren, R., and Katul, G. G.: Finite element tree crown hydrodynamics model (FETCH) using porous media flow within branching elements: A new representation of tree hydrodynamics, Water Resour. Res., 41, 11404, https://doi.org/10.1029/2005WR004181, 2005. a
Bonan, G.: Climate Change and Terrestrial Ecosystem Modeling, Cambridge University Press, Cambridge, UK, https://doi.org/10.1017/9781107339217, 2019. a
Bonan, G. B., Williams, M., Fisher, R. A., and Oleson, K. W.: Modeling stomatal conductance in the earth system: linking leaf water-use efficiency and water transport along the soil–plant–atmosphere continuum, Geosci. Model Dev., 7, 2193–2222, https://doi.org/10.5194/gmd-7-2193-2014, 2014. a, b
Buckley, T. N.: Modeling Stomatal Conductance, Plant Physiol., 174, 572–582, https://doi.org/10.1104/pp.16.01772, 2017. a, b, c
Buckley, T. N.: How do stomata respond to water status?, New Phytol., 224, 21–36, https://doi.org/10.1111/nph.15899, 2019. a, b
Buckley, T. N. and Mott, K. A.: Modelling stomatal conductance in response to environmental factors, Plant Cell Environ., 36, 1691–1699, https://doi.org/10.1111/pce.12140, 2013. a
Budyko, M. I.: Teplovoi Balans Zemnoi Poverkhnosti, Gidrometeoizdat, Leningrad, 1956. a
Christoffersen, B. O., Gloor, M., Fauset, S., Fyllas, N. M., Galbraith, D. R., Baker, T. R., Kruijt, B., Rowland, L., Fisher, R. A., Binks, O. J., Sevanto, S., Xu, C., Jansen, S., Choat, B., Mencuccini, M., McDowell, N. G., and Meir, P.: Linking hydraulic traits to tropical forest function in a size-structured and trait-driven model (TFS v.1-Hydro), Geosci. Model Dev., 9, 4227–4255, https://doi.org/10.5194/gmd-9-4227-2016, 2016. a
Clapp, R. B. and Hornberger, G. M.: Empirical equations for some soil hydraulic properties, Water Resour. Res., 14, 601–604, https://doi.org/10.1029/WR014i004p00601, 1978. a
Collatz, G., Ball, J., Grivet, C., and Berry, J. A.: Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary layer, Agr. Forest Meteorol., 54, 107–136, https://doi.org/10.1016/0168-1923(91)90002-8, 1991. a
Couvreur, V., Ledder, G., Manzoni, S., Way, D. A., Muller, E. B., and Russo, S. E.: Water transport through tall trees: A vertically explicit, analytical model of xylem hydraulic conductance in stems, Plant Cell Environ., 41, 1821–1839, https://doi.org/10.1111/pce.13322, 2018. a
Cowan, I. R.: Transport of Water in the Soil-Plant-Atmosphere System, J. Appl. Ecol., 2, 221–239, https://doi.org/10.2307/2401706, 1965. a
Daly, E., Porporato, A., Rodriguez-Iturbe, I., Daly, E., Porporato, A., and Rodriguez-Iturbe, I.: Coupled Dynamics of Photosynthesis, Transpiration, and Soil Water Balance. Part I: Upscaling from Hourly to Daily Level, J. Hydrometeorol., 5, 546–558, https://doi.org/10.1175/1525-7541(2004)005<0546:CDOPTA>2.0.CO;2, 2004. a
Damour, G., Simonneau, T., Cochard, H., and Urban, L.: An overview of models of stomatal conductance at the leaf level, Plant Cell Environ., 33, 1419–1438, https://doi.org/10.1111/j.1365-3040.2010.02181.x, 2010. a
De Kauwe, M. G., Zhou, S.-X., Medlyn, B. E., Pitman, A. J., Wang, Y.-P., Duursma, R. A., and Prentice, I. C.: Do land surface models need to include differential plant species responses to drought? Examining model predictions across a mesic-xeric gradient in Europe, Biogeosciences, 12, 7503–7518, https://doi.org/10.5194/bg-12-7503-2015, 2015. a
DeLucia, E. H. and Heckathorn, S. A.: The effect of soil drought on water‐use efficiency in a contrasting Great Basin desert and Sierran montane species, Plant Cell Environ., 12, 935–940, https://doi.org/10.1111/j.1365-3040.1989.tb01973.x, 1989. a
Denmead, O. T. and Shaw, R. H.: Availability of Soil Water to Plants as Affected by Soil Moisture Content and Meteorological Conditions 1, Agron. J., 54, 385–390, https://doi.org/10.2134/agronj1962.00021962005400050005x, 1962. a
Egea, G., Verhoef, A., and Vidale, P. L.: Towards an improved and more flexible representation of water stress in coupled photosynthesis–stomatal conductance models, Agr. Forest Meteorol., 151, 1370–1384, https://doi.org/10.1016/J.AGRFORMET.2011.05.019, 2011. a, b
Eller, C. B., Rowland, L., Mencuccini, M., Rosas, T., Williams, K., Harper, A., Medlyn, B. E., Wagner, Y., Klein, T., Teodoro, G. S., Oliveira, R. S., Matos, I. S., Rosado, B. H. P., Fuchs, K., Wohlfahrt, G., Montagnani, L., Meir, P., Sitch, S., and Cox, P. M.: Stomatal optimization based on xylem hydraulics (SOX) improves land surface model simulation of vegetation responses to climate, New Phytol., 226, 1622–1637, https://doi.org/10.1111/nph.16419, 2020. a, b, c, d
Farquhar, G. D., von Caemmerer, S., and Berry, J. A.: A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species, 149, 78–90, https://doi.org/10.1007/BF00386231, 1980. a
Fatichi, S., Pappas, C., and Ivanov, V. Y.: Modeling plant-water interactions: an ecohydrological overview from the cell to the global scale, Wiley Interdisciplinary Reviews: Water, 3, 327–368, https://doi.org/10.1002/wat2.1125, 2016. a
Feddes, R. A. and Raats, P. C.: Parameterizing the soil – water – plant root system, in: Unsaturated-zone modeling: Progress, challenges and applications, chap. 4, Kluwer Academic Publishers, Dordrecht, 95–141, 2004. a
Feddes, R. A., Kowalik, P., Kolinska-Malinka, K., and Zaradny, H.: Simulation of field water uptake by plants using a soil water dependent root extraction function, J. Hydrol., 31, 13–26, https://doi.org/10.1016/0022-1694(76)90017-2, 1976. a
Feddes, R. A., Kowalik, P. J., and Zaradny, H.: Simulation of field water use and crop yield. Simulation monographs, Halsted Press, Wageningen, 1978. a, b
Feng, X.: Marching in step: The importance of matching model complexity to data availability in terrestrial biosphere models, Glob. Change Biol., 26, 3190–3192, https://doi.org/10.1111/gcb.15090, 2020. a
Feng, X., Ackerly, D. D., Dawson, T. E., Manzoni, S., Skelton, R. P., Vico, G., and Thompson, S. E.: The ecohydrological context of drought and classification of plant responses, Ecology Letters, 21, 1723–1736, https://doi.org/10.1111/ele.13139, 2018. a, b
Fisher, J. B., Lee, B., Purdy, A. J., Halverson, G. H., Dohlen, M. B., Cawse-Nicholson, K., Wang, A., Anderson, R. G., Aragon, B., Arain, M. A., Baldocchi, D. D., Baker, J. M., Barral, H., Bernacchi, C. J., Bernhofer, C., Biraud, S. C., Bohrer, G., Brunsell, N., Cappelaere, B., Castro-Contreras, S., Chun, J., Conrad, B. J., Cremonese, E., Demarty, J., Desai, A. R., De Ligne, A., Foltýnová, L., Goulden, M. L., Griffis, T. J., Grünwald, T., Johnson, M. S., Kang, M., Kelbe, D., Kowalska, N., Lim, J.-H., Maïnassara, I., Mccabe, M. F., Missik, J. E. C., Mohanty, B. P., Moore, C. E., Morillas, L., Morrison, R., Munger, J. W., Posse, G., Richardson, A. D., Russell, E. S., Ryu, Y., Sanchez-Azofeifa, A., Schmidt, M., Schwartz, E., Sharp, I., Šigut, L., Tang, Y., Hulley, G., Anderson, M., Hain, C., French, A., Wood, E., Hook, S., Fisher, J. B., Lee, B., Purdy, A. J., Halverson, G. H., Dohlen, M. B., and Fisher, A. L.: ECOSTRESS: NASA's Next Generation Mission to Measure Evapotranspiration From the International Space Station, Water Resour. Res., 56, e2019WR026058, https://doi.org/10.1029/2019WR026058, 2020. a
Fisher, R. A., Koven, C. D., Anderegg, W. R. L., Christoffersen, B. O., Dietze, M. C., Farrior, C. E., Holm, J. A., Hurtt, G. C., Knox, R. G., Lawrence, P. J., Lichstein, J. W., Longo, M., Matheny, A. M., Medvigy, D., Muller-Landau, H. C., Powell, T. L., Serbin, S. P., Sato, H., Shuman, J. K., Smith, B., Trugman, A. T., Viskari, T., Verbeeck, H., Weng, E., Xu, C., Xu, X., Zhang, T., and Moorcroft, P. R.: Vegetation demographics in Earth System Models: A review of progress and priorities, Glob. Change Biol., 24, 35–54, https://doi.org/10.1111/gcb.13910, 2018. a
FLUXNET2015: Dataset, available at: https://fluxnet.org/data/fluxnet2015-dataset/, last access: 3 January 2019. a, b
Franks, S. J., Weber, J. J., and Aitken, S. N.: Evolutionary and plastic responses to climate change in terrestrial plant populations, Evol. Appl., 7, 123–139, https://doi.org/10.1111/eva.12112, 2014. a
Gardner, W. R.: Dynamic aspects of water availability to plants, Soil Sci., 89, 63–73, https://doi.org/10.1097/00010694-196002000-00001, 1960. a, b
Goudriaan, J. and van Laar, H. H.: Modelling potential crop growth processes: textbook with exercises, Springer Science and Business Media Dordrecht, Wageningen, first edn., https://doi.org/10.1007/978-94-011-0750-1, 1994. a
Grossiord, C., Buckley, T. N., Cernusak, L. A., Novick, K. A., Poulter, B., Siegwolf, R. T. W., Sperry, J. S., and McDowell, N. G.: Plant responses to rising vapor pressure deficit, New Phytol., 226, 1550–1566, https://doi.org/10.1111/nph.16485, 2020. a
Irvine, J., Law, B. E., Martin, J. G., and Vickers, D.: Interannual variation in soil CO2 efflux and the response of root respiration to climate and canopy gas exchange in mature ponderosa pine, Glob. Change Biol., 14, 2848–2859, https://doi.org/10.1111/j.1365-2486.2008.01682.x, 2008. a
Jarvis, P.: The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field, Philos. T. Roy. Soc. Lond., 273, 593–610, 1976. a
Jarvis, P. G. and McNaughton, K. G.: Stomatal Control of Transpiration: Scaling Up from Leaf to Region, Adv. Ecol. Res., 15, 1–49, https://doi.org/10.1016/S0065-2504(08)60119-1, 1986. a
Katul, G. G., Oren, R., Manzoni, S., Higgins, C., and Parlange, M. B.: Evapotranspiration: A process driving mass transport and energy exchange in the soil-plant-atmosphere-climate system, Rev. Geophys., 50, RG3002, https://doi.org/10.1029/2011RG000366, 2012. a
Kennedy, D., Swenson, S., Oleson, K. W., Lawrence, D. M., Fisher, R., Lola da Costa, A. C., and Gentine, P.: Implementing Plant Hydraulics in the Community Land Model, Version 5, J. Adv. Model. Earth Sy., 11, 485–513, https://doi.org/10.1029/2018MS001500, 2019. a, b, c, d, e, f, g, h
Klein, T.: The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours, Funct. Ecol., 28, 1313–1320, https://doi.org/10.1111/1365-2435.12289, 2014. a, b
Kowalczyk, E. A., Wang, Y. P., Law, R. M., Davies, H. L., Mcgregor, J. L., and Abramowitz, G.: The CSIRO Atmosphere Biosphere Land Exchange (CABLE) model for use in climate models and as an offline model, Tech. rep., Commonwealth Scientific and Industrial Research Organisation, available at: http://www.cmar.csiro.au/e-print/open/kowalczykea_2006a.pdf (last access: 4 December 2018), 2006. a
Kroes, J. G., van Dam, J., Bartholomeus, R., Groenendijk, P., Heinen, M., Hendriks, R., Mulder, H., Supit, I., and van Walsum, P.: SWAP version 4: Theory description and user manual, Tech. rep., Wageningen Environmental Research, Wageningen, ISSN 1566-7197, 2017. a, b
Law, B. E.: AmeriFlux US-Me2 Metolius mature ponderosa pine, Ver. 16-5, Ameriflux AMP [data set], https://doi.org/10.17190/AMF/1246076, 2021. a, b
Lin, C., Gentine, P., Huang, Y., Guan, K., Kimm, H., and Zhou, S.: Diel ecosystem conductance response to vapor pressure deficit is suboptimal and independent of soil moisture, Agr. Forest Meteorol., 250–251, 24–34, https://doi.org/10.1016/J.AGRFORMET.2017.12.078, 2018. a
Liu, Y., Kumar, M., Katul, G. G., Feng, X., and Konings, A. G.: Plant hydraulics accentuates the effect of atmospheric moisture stress on transpiration, Nat. Clim. Change, 10, 691–695, https://doi.org/10.1038/s41558-020-0781-5, 2020. a, b
Manabe, S.: Climate and the Ocean Circulation: I. The Atmospheric Circulation and the Hydrology of the Earth's Surface, Mon. Weather Rev., 97, 739–774, https://doi.org/10.1175/1520-0493(1969)097<0739:CATOC>2.3.CO;2, 1969. a
Medlyn, B. E., Duursma, R. A., Eamus, D., Ellsworth, D. S., Prentice, I. C., Barton, C. V. M., Crous, K. Y., De Angelis, P., Freeman, M., and Wingate, L.: Reconciling the optimal and empirical approaches to modelling stomatal conductance, Glob. Change Biol., 17, 2134–2144, https://doi.org/10.1111/j.1365-2486.2010.02375.x, 2011. a
Medlyn, B. E., De Kauwe, M. G., Zaehle, S., Walker, A. P., Duursma, R. A., Luus, K., Mishurov, M., Pak, B., Smith, B., Wang, Y.-P., Yang, X., Crous, K. Y., Drake, J. E., Gimeno, T. E., Macdonald, C. A., Norby, R. J., Power, S. A., Tjoelker, M. G., and Ellsworth, D. S.: Using models to guide field experiments: a priori predictions for the CO2 response of a nutrient- and water-limited native Eucalypt woodland, Glob. Change Biol., 22, 2834–2851, https://doi.org/10.1111/gcb.13268, 2016. a, b
Medvigy, D., Wofsy, S. C., Munger, J. W., Hollinger, D. Y., and Moorcroft, P. R.: Mechanistic scaling of ecosystem function and dynamics in space and time: Ecosystem Demography model version 2, J. Geophys. Res.-Biogeo., 114, G01002, https://doi.org/10.1029/2008JG000812, 2009. a
Mencuccini, M., Manzoni, S., and Christoffersen, B. O.: Modelling water fluxes in plants: from tissues to biosphere, New Phytol., 222, 1207–1222, https://doi.org/10.1111/nph.15681, 2019. a
Novick, K. A., Ficklin, D. L., Stoy, P. C., Williams, C. A., Bohrer, G., Oishi, A. C., Papuga, S. A., Blanken, P. D., Noormets, A., Sulman, B. N., Scott, R. L., Wang, L., and Phillips, R. P.: The increasing importance of atmospheric demand for ecosystem water and carbon fluxes, Nat. Clim. Change, 6, 1023–1027, https://doi.org/10.1038/nclimate3114, 2016. a
Oleson, K. W., Lead, D. M. L., Bonan, G. B., Drewniak, B., Huang, M., Koven, C. D., Levis, S., Li, F., Riley, W. J., Subin, Z. M., Swenson, S. C., Thornton, P. E., Bozbiyik, A., Fisher, R., Heald, C. L., Kluzek, E., Lamarque, J.-F., Lawrence, P. J., Leung, L. R., Lipscomb, W., Muszala, S., Ricciuto, D. M., Sacks, W., Sun, Y., Tang, J., and Yang, Z.-L.: Technical Description of the version 5.0 of the Community Land Model (CLM), Tech. rep., National Center for Atmospheric Research, Boulder, available at: http://www.cesm.ucar.edu/models/cesm2/land/CLM50_Tech_Note.pdf, last access: 11 December 2018. a, b
Pammenter, N. W. and Willigen, C. V.: A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation, Tree Physiol., 18, 589–593, https://doi.org/10.1093/treephys/18.8-9.589, 1998. a
Paschalis, A., Fatichi, S., Zscheischler, J., Ciais, P., Bahn, M., Boysen, L., Chang, J., De Kauwe, M., Estiarte, M., Goll, D., Hanson, P. J., Harper, A. B., Hou, E., Kigel, J., Knapp, A. K., Larsen, K. S., Li, W., Lienert, S., Luo, Y., Meir, P., Nabel, J. E., Ogaya, R., Parolari, A. J., Peng, C., Peñuelas, J., Pongratz, J., Rambal, S., Schmidt, I. K., Shi, H., Sternberg, M., Tian, H., Tschumi, E., Ukkola, A., Vicca, S., Viovy, N., Wang, Y. P., Wang, Z., Williams, K., Wu, D., and Zhu, Q.: Rainfall manipulation experiments as simulated by terrestrial biosphere models: Where do we stand? Global Change Biol., 26, 3336–3355, https://doi.org/10.1111/gcb.15024, 2020. a, b, c, d
Pastorello, G., Trotta, C., Canfora, E., et al.: The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data, Scientific Data, 7, 225, https://doi.org/10.1038/s41597-020-0534-3, 2020. a, b
Powell, T. L., Galbraith, D. R., Christoffersen, B. O., Harper, A., Imbuzeiro, H. M., Rowland, L., Almeida, S., Brando, P. M., da Costa, A. C. L., Costa, M. H., Levine, N. M., Malhi, Y., Saleska, S. R., Sotta, E., Williams, M., Meir, P., and Moorcroft, P. R.: Confronting model predictions of carbon fluxes with measurements of Amazon forests subjected to experimental drought, New Phytol., 200, 350–365, https://doi.org/10.1111/nph.12390, 2013. a, b, c, d
Prentice, I. C., Liang, X., Medlyn, B. E., and Wang, Y.-P.: Reliable, robust and realistic: the three R's of next-generation land-surface modelling, Atmos. Chem. Phys., 15, 5987–6005, https://doi.org/10.5194/acp-15-5987-2015, 2015. a
Razavi, S., Sheikholeslami, R., Gupta, H. V., and Haghnegahdar, A.: VARS-TOOL: A toolbox for comprehensive, efficient, and robust sensitivity and uncertainty analysis, Environ. Modell. Softw., 112, 95–107, https://doi.org/10.1016/j.envsoft.2018.10.005, 2019. a
Restrepo-Coupe, N., Levine, N. M., Christoffersen, B. O., Albert, L. P., Wu, J., Costa, M. H., Galbraith, D., Imbuzeiro, H., Martins, G., da Araujo, A. C., Malhi, Y. S., Zeng, X., Moorcroft, P., and Saleska, S. R.: Do dynamic global vegetation models capture the seasonality of carbon fluxes in the Amazon basin? A data-model intercomparison, Glob. Change Biol., 23, 191–208, https://doi.org/10.1111/gcb.13442, 2017. a, b
Rogers, A., Medlyn, B. E., Dukes, J. S., Bonan, G., von Caemmerer, S., Dietze, M. C., Kattge, J., Leakey, A. D. B., Mercado, L. M., Niinemets, Ü., Prentice, I. C., Serbin, S. P., Sitch, S., Way, D. A., and Zaehle, S.: A roadmap for improving the representation of photosynthesis in Earth system models, New Phytol., 213, 22–42, https://doi.org/10.1111/nph.14283, 2017. a
Sabot, M. E. B., De Kauwe, M. G., Pitman, A. J., Medlyn, B. E., Verhoef, A., Ukkola, A. M., and Abramowitz, G.: Plant profit maximization improves predictions of European forest responses to drought, New Phytol., 226, 1638–1655, https://doi.org/10.1111/nph.16376, 2020. a, b, c, d
Schwarz, P. A., Law, B. E., Williams, M., Irvine, J., Kurpius, M., and Moore, D.: Climatic versus biotic constraints on carbon and water fluxes in seasonally drought-affected ponderosa pine ecosystems, Global Biogeochem. Cy., 18, GB4007, https://doi.org/10.1029/2004GB002234, 2004. a
Sitch, S., Huntingford, C., Gedney, N., Levy, P. E., Lomas, M., Piao, S. L., Betts, R., Ciais, P., Cox, P. M., Friedlingstein, P., Jones, C. D., Prentice, I. C., and Woodward, F. I.: Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs), Glob. Change Biol., 14, 2015–2039, https://doi.org/10.1111/j.1365-2486.2008.01626.x, 2008. a
Sloan, B. P.: LSM for “Plant hydraulic transport controls transpiration response to soil water stress”, Zenodo [code], https://doi.org/10.5281/zenodo.5129247, 2021. a, b
Sperry, J. S. and Love, D. M.: What plant hydraulics can tell us about responses to climate-change droughts, New Phytol., 207, 14–27, https://doi.org/10.1111/nph.13354, 2015. a
Sperry, J. S., Adler, F. R., Campbell, G. S., and Comstock, J. P.: Limitation of plant water use by rhizosphere and xylem conductance: Results from a model, Plant Cell Environ., 21, 347–359, https://doi.org/10.1046/j.1365-3040.1998.00287.x, 1998. a, b
Trugman, A. T., Medvigy, D., Mankin, J. S., and Anderegg, W. R.: Soil Moisture Stress as a Major Driver of Carbon Cycle Uncertainty, Geophys. Res. Lett., 45, 6495–6503, https://doi.org/10.1029/2018GL078131, 2018. a, b, c, d, e
Ukkola, A. M., De Kauwe, M. G., Pitman, A. J., Best, M. J., Abramowitz, G., Haverd, V., Decker, M., and Haughton, N.: Land surface models systematically overestimate the intensity, duration and magnitude of seasonal-scale evaporative droughts, Environ. Res. Lett., 11, 104012, https://doi.org/10.1088/1748-9326/11/10/104012, 2016. a, b
Verhoef, A. and Egea, G.: Modeling plant transpiration under limited soil water: Comparison of different plant and soil hydraulic parameterizations and preliminary implications for their use in land surface models, Agr. Forest Meteorol., 191, 22–32, https://doi.org/10.1016/J.AGRFORMET.2014.02.009, 2014. a, b
Williams, M., Law, B. E., Anthoni, P. M., and Unsworth, M. H.: Use of a simulation model and ecosystem flux data to examine carbon-water interactions in ponderosa pine, Tree Physiol., 21, 287–298, https://doi.org/10.1093/treephys/21.5.287, 2001. a
Wolf, A., Anderegg, W. R. L., and Pacala, S. W.: Optimal stomatal behavior with competition for water and risk of hydraulic impairment., P. Natl. Acad. Sci. USA, 113, E7222–E7230, https://doi.org/10.1073/pnas.1615144113, 2016. a
Xu, X., Medvigy, D., Powers, J. S., Becknell, J. M., and Guan, K.: Diversity in plant hydraulic traits explains seasonal and inter-annual variations of vegetation dynamics in seasonally dry tropical forests, New Phytol., 212, 80–95, https://doi.org/10.1111/nph.14009, 2016. a, b
Yang, S. J. and de Jong, E.: Effect of Aerial Environment and Soil Water Potential on the Transpiration and Energy Status of Water in Wheat Plants 1, Agron. J., 64, 574–578, https://doi.org/10.2134/agronj1972.00021962006400050006x, 1972. a
Zhang, Q., Manzoni, S., Katul, G., Porporato, A., and Yang, D.: The hysteretic evapotranspiration-papor pressure deficit relation, J. Geophys. Res.-Biogeo., 119, 125–140, https://doi.org/10.1002/2013JG002484, 2014. a
Zhou, S., Duursma, R. A., Medlyn, B. E., Kelly, J. W., and Prentice, I. C.: How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress, Agr. Forest Meteorol., 182–183, 204–214, https://doi.org/10.1016/J.AGRFORMET.2013.05.009, 2013. a





