the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Coffee and shade trees show complementary use of soil water in a traditional agroforestry ecosystem
Lyssette Elena Muñoz-Villers
Josie Geris
María Susana Alvarado-Barrientos
Friso Holwerda
Todd Dawson
Globally, coffee has become one of the most sensitive commercial crops, being affected by climate change. Arabica coffee (Coffea arabica) grows in traditionally shaded agroforestry systems in tropical regions and accounts for ∼70 % of coffee production worldwide. Nevertheless, the interaction between plant and soil water sources in these coffee plantations remains poorly understood. To investigate the functional response of dominant shade tree species and coffee (C. arabica var. typica) plants to different soil water availability conditions, we conducted a study during near-normal and more pronounced dry seasons (2014 and 2017, respectively) and a wet season (2017) in a traditional coffee plantation in central Veracruz, Mexico. For the different periods, we specifically investigated the variations in water sources and root water uptake via MixSIAR mixing models that use δ18O and δ2H stable isotope composition of rainfall, plant xylem and soil water. To further increase our mechanistic understanding of root activity, the distribution of below-ground biomass and soil macronutrients was also examined and considered in the model as prior information. Results showed that, over the course of the two investigated dry seasons, all shade tree species (Lonchocarpus guatemalensis, Inga vera and Trema micrantha) relied, on average, on water sources from intermediate (>15 to 30 cm depth: 58± 18 % SD) and deep soil layers (>30 to 120 cm depth: 34±21 %), while coffee plants used much shallower water sources (<5 cm depth: 42±37 % and 5–15 cm depth: 52±35 %). In addition, in these same periods, coffee water uptake was influenced by antecedent precipitation, whereas trees showed little sensitiveness to antecedent wetness. Our findings also showed that during the wet season coffee plants substantially increased the use of near-surface water (+56 % from <5 cm depth), while shade trees extended the water acquisition to much shallower soil layers (+19 % from <15 cm depth) in comparison to drier periods. Despite the plasticity in root water uptake observed between canopy trees and coffee plants, a complementary use of soil water prevailed during the dry and wet seasons investigated. However, more variability in plant water sources was observed among species in the rainy season when higher soil moisture conditions were present and water stress was largely absent.
- Article
(5722 KB) - Full-text XML
-
Supplement
(760 KB) - BibTeX
- EndNote
Coffee agroforestry systems are highly valued because of their ecological, environmental, economic and social benefits (Mas and Dietsch, 2004; Perfecto et al., 2007; Tscharntke et al., 2011). Moreover, shade coffee of the species Arabica (Coffea arabica) accounts for ∼70 % of the total coffee production (USDA, 2018). Although Arabica coffee is mainly grown in tropical montane regions, it is cultivated under a wide range of climatic and soil conditions (Jha et al., 2014). Coffee Arabica plantations can be broadly classified as traditional or modern coffee systems, according to vegetation composition and structure and management practices (Moguel and Toledo, 1999). In the traditional systems, coffee plants are cultivated under a diverse canopy of native and/or introduced shade tree species. In contrast, monoculture coffee plantations exemplify the modern cultivation scheme, in which the shade is provided by a single commercial tree species. The use of agrochemicals is also typically required in this type of plantation (Moguel and Toledo, 1999).
Until recently, the vast majority of Arabica coffee was cultivated in traditionally managed shaded coffee plantations, which have lower production costs and enhanced biodiversity, carbon sequestration, soil fertility and biological pest control in comparison to modern systems (Greenberg et al., 1997; Perfecto et al., 2002; Kellermann et al., 2008). However, coffee management practices have become more intensive, promoting the replacement of native trees with fast-growing monospecific timber species (i.e., Cedrela odorata, Eucalyptus deplupta, Hevea brasilensis) (Nath et al., 2011).
Growing a crop in association with shade trees inevitably leads to some degree of competition for the above-ground (light) and below-ground (water and nutrients) resources (Monteith et al., 1991). In an agroforestry system, the outcome of competition for light is relatively predictable due to the hierarchical structure of the canopy (i.e., shade trees intercept part of the sunlight, thereby reducing the amount available for the understory crop). Conversely, competitive interactions for below-ground resources can be much more diverse and complex. The central hypothesis of agroforestry underscores that crops and trees are complementary in their use of soil water (Cannell et al., 1996); however, the degree to which this occurs will be largely controlled by the spatial and temporal patterns of resource availability, root distribution and root activity, which in turn depend on factors such as climate, soil conditions, crop and tree species, and plantation age, density and management practices (Beer et al., 1998; Lehmann, 2003; van Noordwijk et al., 2015). In addition, below-ground competitive interactions for water and/or nutrients are much more difficult to elucidate than above-ground relationships. So far, the most common approach is to measure the distribution of root abundance of crops and trees and examine to what extent they overlap or are separated (e.g., Schaller et al., 2003; van Kanten et al., 2005). An important limitation of this method is, however, that the spatial distribution of roots does not always mirror the actual resource capture along the soil profile (Dawson et al., 2002; Lehmann, 2003). Another approach is to examine the vertical patterns of soil water (Cannavo et al., 2011; Padovan et al., 2015) or nutrient (Schroth et al., 2000, cited in Lehmann, 2003) depletion. However, these methods are problematic because they cannot provide information on whether resource depletion is caused by the crop, the trees, or both (Cannavo et al., 2011; Padovan et al., 2015). Recently, the use of hydrogen (δ2H) and oxygen (δ18O) water stable isotope techniques in combination with mixing models based on Bayesian theory has proved to be a powerful tool for quantifying the proportions and probability distributions of different water sources to plant uptake across different ecosystems and regions (Barbeta et al., 2015; Beyer et al., 2018; Penna et al., 2018), with the potential to largely overcome the above-mentioned limitations (Dawson et al., 2002; Lehmann, 2003; van Noordwijk et al., 2015). Although rarely implemented, including nutrient and root distribution data along the soil profile to inform these models could provide more comprehensive insights into depth of plant water uptake (cf. Muñoz-Villers et al., 2018).
To date, research into plant–soil interactions and plant water source partitioning in coffee agroforestry systems has been extremely scarce. To our knowledge, only five studies have investigated the water sources of shade trees and coffee shrubs using either information on the isotopic composition of plant xylem and bulk soil water (Wu et al., 2016), soil water depletion (Cannavo et al., 2011; Padovan et al., 2015) or root distribution (Schaller et al., 2003; van Kanten et al., 2005). Moreover, all these studies have been carried out in intensive monospecific plantations characterized by high coffee planting densities (∼4000–5000 shrubs per hectare), and low density (∼150–280 trees per hectare) and very low diversity (one to two species) of shade trees. While recognizing the limitations of some of the methods used in these previous studies, the available information suggests that competition for water between coffee and trees can be strong at sites with a pronounced seasonal dry period (Padovan et al., 2015; Wu et al., 2016), while it seems to be virtually absent at sites with no or a relatively short dry season (Schaller et al., 2003; Cannavo et al., 2011). Further, although most coffee roots are usually located in the upper soil layers (<30 cm depth; van Kanten et al., 2005, and references therein), the plant and soil interactions for water during the dry season seem to occur below the main crop rooting zone (>30 cm depth) (Wu et al., 2016). The latter reflects the ability of coffee to develop an extensive root system and to increase the root water uptake at greater soil depths once the available water has been depleted in shallower layers (Huxley et al., 1974, cited in Lehmann, 2003).
Currently, we lack information on plant water sources in traditional shade coffee plantations. In these agroforestry systems, the higher density and diversity of shade trees could potentially lead to stronger and more diverse tree–crop interactions (van Noordwijk et al., 2015). On the other hand, the dense tree canopy reduces light availability and hence limits coffee water use. This could lead to a lower soil water demand and thus increased plant water availability during the dry season.
Further, ecohydrological research in these shade coffee systems is becoming increasingly important since trees have been promoted as a strategy for mitigating and adapting to future climate (Schroth et al., 2009; Vaast et al., 2016; Rice, 2018). Shaded coffee plantations store more carbon than sun-grown coffee systems, thereby contributing to the reduction of greenhouse gases (Vaast et al., 2016; Rice, 2018, and references therein). In addition, the tree canopy provides some level of protection against the rising mean and maximum air temperatures (Baker and Haggar, 2007; Schroth et al., 2009; Vaast et al., 2016), which in recent modeling studies have been pointed out as the key climatic changes affecting coffee growth, yield and quality (Schroth et al., 2009; Baca et al., 2014; Bunn et al., 2015). Although there are important differences across sites, rainfall is also predicted to decrease and become more variable in many of the world's coffee-growing regions. For example, Giorgi (2006) estimated that rainfall will decrease by about 17 % (per 100 years) during the dry season and by about 9 % during the wet season in Mexico and central America. Similarly, predictions by Karmalkar et al. (2011) for the same regions pointed out changes in rainfall of −24 % to +8 % (per 100 years) during the dry season and of −39 % to −1 % during the wet season. As such, if warming is accompanied by decreases in rainfall, this could lead to, or exacerbate, competition for water sources between coffee shrubs and shade trees (Baker and Haggar, 2007), which in turn could affect the long-term sustainability of these agroecosystems.
Mexico is among the largest shade coffee producers in the world, and the central region of Veracruz constitutes the second most important coffee zone in the country. In this area, we selected a representative traditional shade coffee plantation to investigate plant water sources of dominant shade tree species and coffee (C. arabica var. typica) shrubs under different conditions of soil water availability. During near-normal and more pronounced dry seasons (2014 and 2017, respectively) and a wet season (2017), variations in depth of plant water uptake were examined using the stable isotopic composition (δ18O and δ2H) of rainfall, plant xylem and soil water in combination with a Bayesian mixing model (MixSIAR), along with microclimatic and soil moisture measurements. To further increase our understanding of root activity and water uptake, the distribution of roots and macronutrients along the soil profile was also examined and considered in the mixing model as prior information. Specifically, we addressed the following questions.
-
Does a complementary water use strategy between shade trees and coffee shrubs prevail over competition in a traditional shaded agroforestry system?
-
Does competition exist for water sources among tree and coffee species during more pronounced dry periods?
-
What are the seasonal patterns in plant–water source partitioning?
2.1 Study site
The research was carried out in La Orduña coffee plantation (∼100 ha) located on a flat plateau at an elevation of 1210 m a.s.l. on the eastern slopes of the Cofre de Perote mountain (19∘28′ N, 96∘56′ W) in central Veracruz State, Mexico (Fig. 1). The coffee plantations in this region occur between elevations of 1000 and 1350 m a.s.l. (Marchal and Palma, 1985; Hernández-Martínez et al., 2013).
Figure 1Study site location in the municipality of Coatepec, Veracruz, Mexico. Source: QuickBird Satellite Image (Digital Globe, 2010) © DigitalGlobe, Inc.
The climate is classified as temperate humid with abundant rains during the summer (García, 1988). Two distinct seasons can be distinguished: (1) a wet season (May–October), during which rainfall is associated primarily with cumulus and cumulonimbus clouds formed during convective and orographic uplift of the moist maritime air masses brought in by the easterly trade winds; and (2) a (relatively) dry season (November–April), during which most rainfall falls from stratus clouds associated with the passage of cold fronts (Báez et al., 1997). Mean annual rainfall measured nearby the study site during the period 1971–2000 was 1765 mm, with on average 389 mm falling during the dry season and 1376 mm falling during the wet season (SMN, 2014). Mean annual temperature over this period was 19.5 ∘C, with minimum and maximum monthly average values of 15.5 and 22.5 ∘C observed in January and May, respectively (SMN, 2014). Annual potential evapotranspiration (ET0) is about 1120 mm (Holwerda et al., 2013).
The investigated shade coffee plantation is a so-called traditional commercial polyculture system (sensu Moguel and Toledo, 1999) which was established more than 80 years ago. The tree canopy was diverse and consisted predominantly of the species Inga spp., Citrus spp., Lonchocarpus guatemalensis, Trema micrantha and Enterolobium cyclocarpum (Holwerda et al., 2016). The shade trees were planted at a density of ca. 500 ha−1 and currently form a canopy of about 14 m high. The Arabica coffee plants were of the variety typica. Typica – a tall cultivar of Coffea arabica – was the first coffee variety that arrived in Mexico from Ethiopia (Renard, 2010); it has bronze-tipped young leaves and the berries are large. Plants of the typica variety are tolerant to conditions of low soil fertility and drought but vulnerable to most pests and diseases (Escamilla et al., 2005). In the study site, this cultivar was planted approximately 20 years ago at a density of about 1700 shrubs per hectare, currently having an average height of ∼2 m. In this region, the coffee flowering occurs in March or April, fruit development between May and October, and ripening and harvest between October and February (Villers et al., 2009). The management of the plantation involves weed control practices and selective pruning of mature coffee plants and shade trees at irregular times once every ∼7 years (cf. Hernández-Martínez et al., 2009). No pruning activities occurred during or in between our study periods. A photograph of the coffee plantation is provided in the Supplement.
The soil type is an Andic Acrisol derived from volcanic ashes. Soil profiles (∼150 cm) are multilayered (A, B1/BT and BC) and have clay (∼65 %) as the dominant texture across all layers. A general description of the soil profile showed a dark brown to dark yellowish brown, clay silty organic A horizon (0–20 cm) overlying a dark yellowish brown, clay silty sand B1/BT horizon (20–135 cm), followed by a dark yellowish brown, clay sandy BC horizon (>135 cm). Average soil bulk densities and porosities were 1.2 g cm−3 and 63 %, respectively, along the A and B horizons (Holwerda et al., 2013). The underlying material consists of deeply weathered old lava and sandy–gravelly pyroclastic flow deposits (Rodríguez et al., 2010). Soils were mostly covered by a thin (1–2 cm) but continuous layer of litter.
2.2 Hydrometeorological measurements
During the study period, rainfall and microclimate conditions were continuously monitored above the canopy in an 18 m high tower, located in the southwestern part of the coffee plantation. Rainfall (P, mm) was measured using a TR-525 M tipping bucket rain gauge (Texas Electronics, USA). Temperature (T, ∘C) and relative humidity (RH, %) were measured using a HC2-S3 probe (Rotronic, USA). Data were recorded every 30 s, and accumulated (P) or averaged values (all other parameters) were stored at 5 min intervals using a CR1000 datalogger (Campbell Scientific Ltd., USA).
2.3 Isotope sampling
To examine the water sources of overstory shade trees and understory coffee shrubs, plant tissue and soil samples were collected for isotope analysis at the middle (23 January) and end (11 and 26 April) of the 2014 dry season. In 2017, the dry season was warmer and drier, offering the opportunity to examine the vegetation responses to more pronounced dry conditions. Therefore, a second sampling campaign was carried out to collect plant and bulk soil samples at the middle (27 February), end (5 April) and late end (20 May) of the 2017 dry season. Another sampling was carried out in the middle of the 2017 wet season (4 August) to evaluate plant–soil water uptake patterns at higher soil water availability conditions.
Table 1Characteristics of the shade trees and coffee plants sampled for water isotope analysis during 2014 and 2017. Numbers between parentheses are the standard deviation.
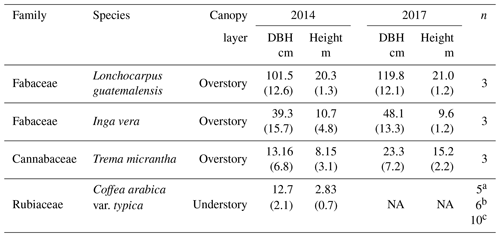
a Number of individuals sampled each time in the 2014 dry season; b number of individuals sampled each time in the 2017 dry season; and c number of individuals sampled in the 2017 wet season. NA means not available.
In all seven samplings, xylem samples were obtained from three individuals of each of the three dominant shade tree species (Lonchocarpus guatemalensis, Inga vera and Trema micrantha) by extracting ∼5–6 cm cores using a Pressler increment borer inserted at 1.2 m above ground (n=60 samples of trees in total). On each occasion, xylem samples were taken from the same individuals but from various aspects of the trunk. The bark was immediately removed after core extraction to avoid contamination of phloem water. For the coffee plants, samples were obtained from ∼6 cm segments of mature suberized branches that were cut near the main stem of several shrubs each time. The bark (∼1 mm thick) and cambium were not stripped from the coffee branches, to avoid exposure of the samples to evaporation. All coffee plants were sampled randomly (n=40 samples of coffee shrubs in total). During the 2014 and 2017 dry seasons, sampling of coffee shrubs involved five to six individuals each time. Since only one sampling occasion was performed during the 2017 wet season, a larger number of individuals (10) was sampled to reduce the uncertainties associated with different sampling sizes between wet and dry seasons, respectively. For each tree, we measured diameter at breast height (DBH) and height, and for the coffee plants the diameter of the main stem was measured below its bifurcation in small branches (Table 1).
Bulk soil samples were collected at three locations and at depths of 5, 15, 30, 60, 90 and 120 cm, using a hand auger (n=126 samples of soil in total). Auger sampling points were located so that each of the sampled shade trees and coffee plants had one soil sampling point within a 3 m radius.
Samples of xylem and bulk soil were collected during the morning and early afternoon (between 08:30 and 13:30 LT), and each sampling campaign was preceded by at least 6 d up to 22 d without or with minimum accumulated rainfall (<5 mm). All xylem and soil samples were collected quickly and carefully and stored in water-tight vials to avoid any evaporation (see section below).
To establish the local meteoric water line and compare soil water sources with recent rainfall, bulk samples of rainfall (n=80 in total) were collected weekly at a nearby (∼5 km) meteorological station over the course of the two years studied (November 2013–October 2014 and November 2016–October 2017) as part of a long-term isotope sampling of precipitation (cf. Muñoz-Villers et al., 2018).
2.4 Isotope collection and analysis
Samples of precipitation, plant xylem and bulk soil for isotope analysis were collected in 30 mL borosilicate glass vials sealed with polycone caps to prevent evaporation. All samples were refrigerated until extraction and analysis at the Center of Stable Isotope Biogeochemistry (CSIB) at the University of California-Berkeley, USA.
Xylem and soil samples were extracted using cryogenic vacuum distillation (temperature: 100±1.1 ∘C, vacuum: 3±1.5 Pa and time: 60–70 min) following the method of West et al. (2006). The δ2H and δ18O isotopic compositions of extracted water samples were determined using an isotope-ratio mass spectrometer (Thermo Delta Plus XL, Thermo Fisher Scientific, USA). The analytical precision of the instrument was ±0.60 ‰ (1 SD) for δ2H and ±0.12 ‰ (1 SD) for δ18O. Samples of precipitation were analyzed for δ2H and δ18O using a laser water isotope analyzer (L2140-i) from Picarro Inc. (Santa Clara, CA, USA) at high precision and without Micro-Combustion Module mode. The analytical precision was ±0.65 ‰ (1 SD) and ±0.20 ‰ (1 SD) for δ2H and δ18O, respectively.
The isotope values are expressed in delta notation (‰) relative to Vienna Standard Mean Ocean Water (VSMOW). To evaluate evaporative enrichment in the soil and xylem water isotopes relative to rainfall, we calculated the deuterium-excess parameter (; Dansgaard, 1964).
2.5 Soil sampling and laboratory determinations
To determine volumetric soil water content (SWC), samples were collected at 5, 15, 30, 60, 90 and 120 cm depth from each of the three boreholes excavated during the soil isotope samplings. Soil moisture content was determined gravimetrically and converted to volumetric values by using the bulk density of the soil sample. In addition, to determine the antecedent moisture conditions for the 15 d prior to each sampling date, an antecedent precipitation index (API) was calculated following Viessman et al. (1989).
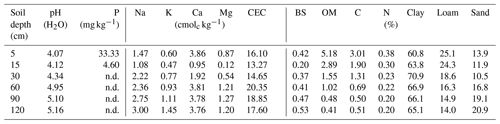
To examine pH and N, P and K macronutrient concentrations along the soil profile, soil samples were collected at 5, 15, 30, 60, 90 and 120 cm depth from each borehole (n=3 samples per soil depth) during three isotope sampling campaigns: 11 April 2014 (dry season), 27 February 2017 (dry season) and 4 August 2017 (wet season). Samples (n=18) for determining other chemical properties were collected at the same depths in soil profiles. All samples were first air-dried and then sieved using 2 mm screens. Soil pH was determined using a glass electrode pH meter in a 1:2 soil : water ratio. Organic matter (OM) was determined by the Walkley–Black method. Total carbon (C) and total nitrogen (N) were measured using a TruSpec dry combustion CN analyzer (LECO, USA). Extractable phosphorus (P) was determined by the Bray I method (Bray and Kurtz, 1945). Exchangeable cations (Ca+, Mg+, K+, Na+) were determined by extracting soil with 1 MNH4OAc (pH 7.0). Ca+ and Mg+ were analyzed using atomic absorption spectrometry and K+ and Na+ were analyzed using flame photometry. Soil cation exchange capacity (CEC) was determined by the ammonium acetate 1N (pH 7.0) method (Van Reeuwijk, 2002) and base saturation (BS) was calculated as the portion of CEC that is occupied by exchangeable bases: (Ca+, Mg+, K+, .
2.6 Root biomass
To examine the root biomass distribution along the soil profile in the study plot, 33 soil cores were collected using 5 cm diameter and 10 cm long samplers. Soil cores were extracted at 5, 20, 40, 60 and 90 cm depth (from 5 to 40 cm: n=9 for each depth, and from 60 to 90 cm: n=3 for each depth). All cores were processed immediately in the laboratory. Soil samples were first sieved using 2 mm screens to separate the bigger roots. Next, the samples were washed using a fine nylon mesh sieve and then separated into diameter classes (<1, 1–2 and >2 mm) and dried at 70 ∘C for 48 h. Root biomass (g m−3) was calculated from the dry weight of the roots and the volume of the core sampler for each class and soil depth. No differentiation between roots of coffee shrubs and shade trees was made.
2.7 Plant water uptake sources and temporal patterns
The MixSIAR Bayesian mixing model framework (Moore and Semmens, 2008; Stock and Semmens, 2017) was used to determine the most likely contributions of water sources for the shade tree species and coffee shrubs sampled over the course of the 2014 (23 January, 11 and 26 April) and 2017 (27 February, 5 April, 20 May) dry seasons and the 2017 wet season (4 August). To assess temporal changes in the different plant water sources, the seven sampling occasions were modeled separately. The mixture data for the model were the mean xylem water isotopic (δ2H and δ18O) composition of the shade tree species and coffee shrubs, changing accordingly with the sampling date. Based on statistical tests, the relative contributions of four potential plant water sources were evaluated and restricted to the following soil groups: near-surface water (<5 cm), shallow (5 to 15 cm), intermediate (>15 to 30 cm) and deep soil water (>30 to 120 cm). For each sampling date, the mean and standard deviation of the soil water isotope (δ2H and δ18O) signatures from the four different grouped soil depths were introduced into the model, all corresponding to the date of xylem tissue collection.
Further, we also considered the use of additional data such as soil macronutrients (N, P, K) and root biomass information to constrain model estimates by specifying an “informative” prior distribution of the soil source proportions (Stock and Semmens, 2017). These data were also grouped into four classes based on the depth of the soil samplings and corresponding largely to the grouping for soil water: near-surface (<5 cm), shallow (5 to 15 cm), intermediate (>15 to 30 cm) and deep (>30 to 120 cm). In addition, the nearest corresponding dry or wet season datasets of soil macronutrients were used according to the date of sampling. More details on the informative prior parametrization are provided in the Supplement. The effect of using these priors (i.e., a weight proportion before considering the isotope data) on the water source distribution was then examined by comparing these with the results of “non-informative” (i.e., all the combinations of proportions of water sources were equally likely) simulations. The results of each of these model runs were accepted based on the examination of Markov chain Monte Carlo convergence using the Gelman–Rubin and Geweke diagnostic tests (Gelman et al., 2014).
Furthermore, the effect of isotope fractionation on the quantification of plant water sources was specifically explored by comparing the results of the informed two-isotope mixing model with those from a mixing model using only one water stable isotope ratio in the MixSIAR Bayesian framework. This approach has been used elsewhere (e.g., Evaristo et al., 2017; Barbeta et al., 2019) to provide some initial insights. Nevertheless, we are aware that the use of a single-isotope ratio approach in a multiple water source model could lead to erroneous results due to the overlap of feasible solutions with poor constraint of uncertainties (see Parnell et al., 2010).
Lastly, the relative contributions of the water sources were compared among shade trees and coffee shrubs across all sampling dates using factorial ANOVA and Tukey's HSD (honestly significant difference) post hoc tests. The analyses were carried out in R Statistical Software version 3.2.4 (R Development Core Team, 2016).
3.1 Hydrometeorological conditions
Precipitation (P) was 1650 mm in the first study year (November 2013–October 2014) and 1423 mm in the second study year (November 2016–October 2017). During the 2013–2014 dry season (November–April), rainfall was 323 mm, and mean daily values of temperature (T) and vapor pressure deficit (VPD) were 17.6±3.0 ∘C and 0.65±0.39 kPa, respectively. The lowest monthly P and the highest T and VPD were observed in April at the end of the dry season (Fig. 2a and b). During the 2016–2017 dry season, rainfall amounted to 235 mm, with the lowest monthly values registered in January and February at the middle of the season (Fig. 2b). Mean daily T was 18.3±2.6 ∘C, with the highest values observed at the end of the dry period. Generally, VPD was high during the entire dry season (0.78±0.46 kPa on average) and reached maximum values in February and May.
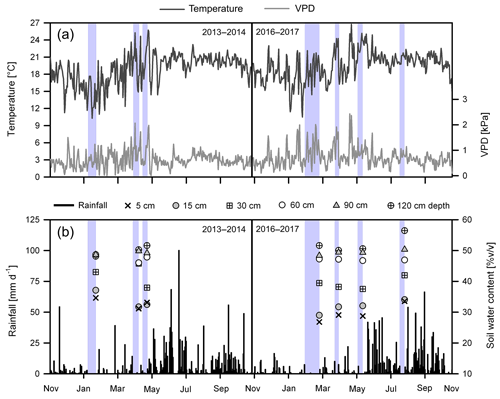
Figure 2(a) Daily mean air temperature and vapor pressure deficit (VPD) and (b) daily total rainfall (P), as measured from November 2013 to October 2014 and from November 2016 to October 2017, and volumetric soil water content (SWC) measured at different depths during the sampling campaigns in the study area; different depths are indicated by the unique symbols shown in the lower panels (the key to the symbols is at the top). The blue-colored areas indicate the 6 to 22 d period of minimum rainfall (<5 mm) preceding the dates of isotope sampling in January (mid dry season) and April (late dry season) of 2014 and in February (mid dry season), April and May (late and end of the dry season), and August (mid wet season) of 2017.
Compared to long-term (1971–2000) climatic records of the region, rainfall in the first study year was very close to the mean annual precipitation of 1765 mm (SMN, 2014). In contrast, the second year was drier (∼300 mm less; −20 %), especially during the dry season, which had about 40 % lower precipitation than the average value of 389 mm. Also, higher mean monthly temperatures (+0.54 ∘C) prevailed across the 2017 dry season in comparison with the 1971–2000 period. Although rainfall during the 2013–2014 dry season was also about 20 % lower than normal, this season was considered to be near average.
Rainfall during the 2017 wet season (May–October) was lower in comparison to 2014 (1188 mm vs. 1326 mm, respectively) (Fig. 2b). Further, the mean air temperature and vapor pressure deficit were slightly higher in the 2017 wet season than in the 2014 wet season (20.7±1.6 ∘C and 0.67±0.25 kPa vs. 20.1±1.5 ∘C and 0.60±0.21 kPa, respectively) (Fig. 2a).
3.2 Soil moisture and antecedent precipitation during sampling campaigns
During the 2014 dry season campaign (January–April), mean soil water content (SWC) was on average 33.8±1.7 % at 5 cm depth, 40.2±14.5 % at 15 cm depth, 38.9±6.4 % at 30 cm depth and 48.3±1.4 % at 60 to 120 cm depth (Fig. 2b). In comparison, SWC in the 2017 dry season campaign (February–May) was lower in the first 30 cm (32.5±3.9 %); meanwhile, water content in the deeper layers was similar (49.0±2.9 %) with respect to the 2014 dry period. In 2014, the lowest SWC values were observed at the end of the dry season (April), whereas the greatest soil moisture depletion in 2017 was registered at the middle of the dry season (February) (Fig. 2b).
During the wet season sampling in August 2017, SWC values at 5 cm (28.2±2.6 %), 15 cm (30.9±4.3 %), 30 cm (38.4±4.8 %) and 60 to 120 cm (49.0±2.9 %) depths were generally higher in comparison to the 2017 dry period (Fig. 2b). Although the 2017 wet season sampling showed slightly lower SWC values in the shallower soil layers in comparison to the 2014 dry season, the SWC values in the deeper layers were higher. For the different samplings, antecedent precipitation conditions (API) were, respectively, 4, 30 and 13 mm for 23 January and 11 and 26 April 2014 and 1, 12, 9 and 43 mm for 27 February, 5 April, 20 May and 4 August 2017.
3.3 Stable isotope composition of waters
Over the study periods, a greater range of variation was found in the rainfall isotope composition of the 2013–2014 year (from −126.7 ‰ to 14.4 ‰ for δ2H; from −17.7 ‰ to 0.0 ‰ for δ18O) in comparison to the 2016–2017 year (from −113.3 ‰ to 15.5 ‰ for δ2H; from −15.9 to 0.0 ‰ for δ18O) (p>0.05) (Fig. 3). Overall, mean dry season rainfall was significantly more enriched than the mean wet season rainfall in δ2H and δ18O (p≤0.001) (Tables 2 and 3). On average, the isotopic compositions of the dry and wet season rainfall were both more depleted during the second study year than during the first study year; thus, the local meteoric water line of 2016–2017 had a slightly steeper slope in comparison to the one for 2013–2014 (Fig. 3). Nevertheless, the range of variation of deuterium-excess values was similar between the years (9 ‰–29 ‰ for the first year vs. 9 ‰–31 ‰ for the second year; Fig. 3), and deuterium-excess values of rainfall within the dry and wet seasons were not statistically different (p≥0.05).
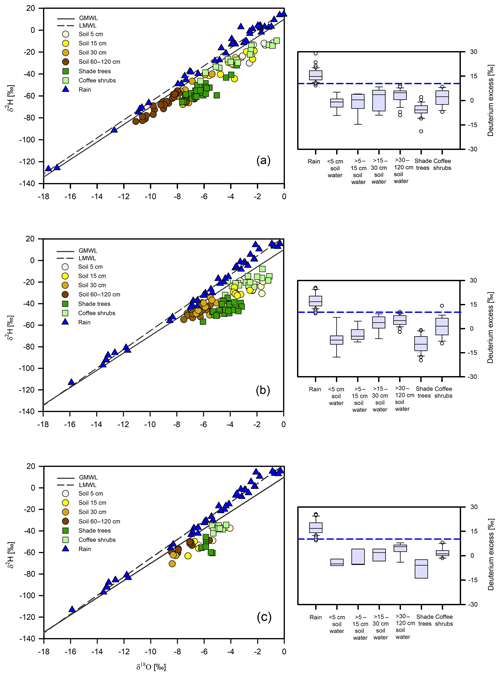
Figure 3(a) Isotope composition of xylem water for shade trees and coffee shrubs, bulk soil at different depths as observed during the three sampling dates (23 January, 11 and 26 April 2014), and isotope values of rainfall during the period December 2013 to November 2014. The dashed line represents the 2013–2014 local meteoric water line (LMWL; ). (b) Isotope composition of xylem water for shade trees and coffee shrubs, bulk soil at different depths during the three sampling dates (27 February, 5 April and 20 May 2017) and isotope values of rainfall during the period December 2016 to November 2017, and (c) isotope composition of xylem water for shade trees and coffee shrubs, bulk soil at different depths during the middle of the 2017 wet season (4 August) and isotope values of rainfall during the period December 2016 to November 2017. The dashed lines in (b) and (c) represent the 2016–2017 local meteoric water line (LMWL; ). The solid line in all the panels represents the global meteoric water line (GMWL; ). The panels on the right show the deuterium-excess values for the plants and soil water sources and rainfall preceding the sampling campaigns. The dashed blue line represents the deuterium-excess value of the GMWL.
Table 2Mean ± (SD) H and O stable isotope composition of 2013–2014 precipitation, tree xylem water and bulk soil water of the 2014 dry season sampling, and corresponding deuterium-excess values (‰).
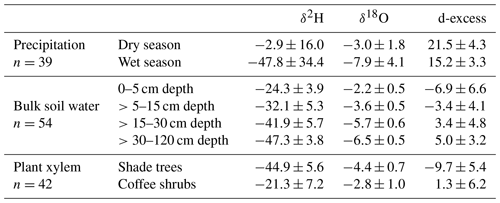
Table 3Mean ± (SD) H and O stable isotope composition of 2016–2017 precipitation, tree xylem water and bulk soil water of 2017 dry season sampling, and corresponding deuterium-excess values (‰).
For all sampling dates, hydrogen and oxygen isotope composition of bulk soil water showed a consistent pattern of increasing isotope depletion with soil depth (Supplement), in which shallower (5–15 cm) soil water was significantly more enriched than intermediate (15–30 cm) and deeper (30–120 cm) soil water layers (p≤0.001) (Tables 2 and 3; Fig. 3). In correspondence, the lowest values of deuterium excess generally characterized the near-surface soil water pool.
For the 2014 dry season samplings, bulk soil ranged from −83.3 ‰ to −11.9 ‰ for δ2H and from −11.1 ‰ to −0.9 ‰ for δ18O (Fig. 3a). For the 2017 dry season samplings, bulk soil water showed a narrower range of variation and more enriched isotope values (from −54.8 ‰ to −19.1 ‰ for δ2H and from −7.5 ‰ to −1.5 ‰ for δ18O) in comparison to 2014 (Fig. 3b). However, statistical differences were only suggested for the intermediate and deeper soil layers in both water isotopes between the two dry seasons investigated (p≤0.001).
In the 2017 wet season sampling, bulk soil isotope composition ranged from −70.5 ‰ to −37.5 ‰ for δ2H and from −8.4 ‰ to −4.1 ‰ for δ18O (Fig. 3c), showing significant differences in the shallow, intermediate and deep soil water pools in comparison to the 2017 dry season (p≤0.001). In all sampling periods, bulk soil water across the different depth groups was isotopically distinct from rainfall during the 2014 and 2017 dry seasons (p≤0.001 for both water isotopes).
Across all sampling periods, xylem water of coffee shrubs was more enriched than that of shade trees (p≤0.001) (Tables 2 and 3; Fig. 3). In the 2014 dry season, xylem water isotope values of shade trees ranged from −65.5 ‰ to −32.1 ‰ for δ2H and from −7.6 ‰ to −3.6 ‰ for δ18O; meanwhile, a larger variation was observed in the xylem water of coffee shrubs (from −46.5 ‰ to −9.6 ‰ for δ2H and from −6.3 ‰ to −0.6 ‰ for δ18O) (p≤0.001) (Fig. 3a). Among tree species, Lonchocarpus guatemalensis showed the most depleted xylem water isotope signature ( ‰ for δ2H and ‰ for δ18O), whereas Inga vera had the most enriched values with a greater range of variation ( ‰ for δ2H and ‰ for δ18O). Statistical tests showed that Inga vera was different from the other tree species in δ18O (p<0.05).
In the 2017 dry season, the isotopic composition of shade trees varied from −56.7 ‰ to −34.5 ‰ for δ2H and from −6.0 to −3.2 ‰ for δ18O; corresponding values for coffee shrubs varied from −39.6 ‰ to −7.8 ‰ for δ2H and from −4.4 ‰ to −1.1 ‰ for δ18O (p≤0.001) (Fig. 3b). In contrast to 2014, L. guatemalensis showed the most enriched isotope value ( ‰ for δ2H and ‰ for δ18O), and I. vera had the most depleted values ( ‰ for δ2H and ‰ for δ18O), with differences being statistically significant for δ2H (p<0.05).
Overall, isotope values of plant xylem water were more enriched during the 2017 dry season than during the 2014 dry season (p≤0.001) (Figs. 3a, b and 4). Deuterium-excess values were also lower in shade trees and coffee shrubs during 2017, indicating a more evaporative signature (Tables 2 and 3; Fig. 3). Plots of δ2H xylem water against height for the individual shade trees and coffee shrubs sampled in both dry seasons are shown in Fig. 4, in which a similar δ2H pattern was displayed between trees and coffee shrubs in the years 2014 and 2017.
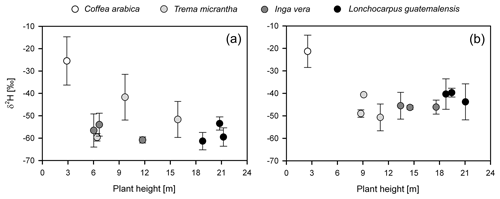
Figure 4Plant height vs. δ2H xylem water for coffee plants and shade tree species corresponding to the (a) 2014 and (b) 2017 dry season samplings.
During the 2017 wet season sampling, δ2H and δ18O values in xylem water of trees and coffee shrubs were more depleted in comparison to the 2017 dry season (p<0.05) (Fig. 3c). The range of variation was from −60.6 ‰ to −45.6 ‰ in δ2H and −6.2 ‰ to −5.4 ‰ in δ18O for trees, and from −42.2 ‰ to −34.4 ‰ in δ2H and −5.4 ‰ to −4.4 ‰ in δ18O for coffee shrubs (p≤0.001).
It was observed that the xylem isotopic composition of all shade trees and coffee plants fell within the range of the soil water sources during the 2014 dry season samplings (Fig. 3a). For the 2017 dry season, we again observed a good isotopic match between the shade tree xylem water and soil water. However, for the coffee plants, the xylem water was more enriched in δ2H in comparison to soil water (Fig. 3b). During the 2017 wet season sampling, a slight enrichment in δ2H was again observed in the xylem water of coffee, while trees showed a good overlap with soil water (Fig. 3c). Based on these results, tests were carried out to specifically evaluate the effects of deuterium fractionation on coffee water sources by running a simple mixing model using only hydrogen isotope ratios in the MixSIAR framework.
3.4 Root biomass and macronutrients along soils profile
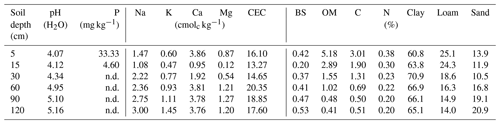
Overall, most roots were concentrated in the first 5 cm of soil with a sharp decline in biomass at 20 cm depth (Fig. 5a). Fine roots (<1 mm) followed by bigger roots (>2 mm) dominated the shallower soil layers (<20 cm); meanwhile, roots in general were scarce at deeper depths (>60 cm). Soil acidity was highest near the surface and decreased gradually with depth (Table 4). Organic matter (OM) and total carbon were also greatest between 5 and 15 cm depth, while values decreased rapidly below ∼30 to 60 cm depth. Although highest concentrations of nitrogen were found in the first 15 cm of soil, values remained relatively high and constant at deeper layers (Fig. 5b). Phosphorus showed its highest concentration at the topsoil with values decreasing sharply below 30 cm depth. In contrast, concentrations of potassium, sodium and magnesium were lowest in the first 15 cm, while maximum values were observed below 90 cm depth. Base saturation (BS) was very low along the soil profile, indicating poor availability of soil macronutrients. Soil cation exchange capacity (CEC) was generally low across depths, indicating little potential for interaction between clay particles and cations.
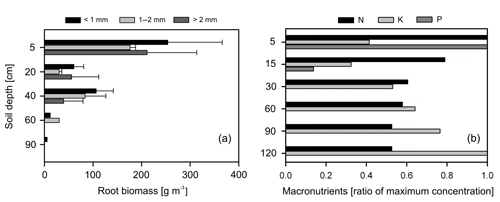
Figure 5(a) Distribution of root biomass for three size classes of roots (different color bars); the error bars represent 1 standard deviation of uncertainty and (b) macronutrient distribution along the soil profile, here normalized and expressed as a ratio to their maximum values (absolute values in Table 4).
3.5 Plant water sources
We found a good agreement between the MixSIAR Bayesian mixing model results using a non-informative and an informative prior distribution (on average 5 % difference across all xylem water contributing sources; p>0.05). This indicates that the independent distribution (soil macronutrients and root data) set a priori to optimize source proportion estimates (informative approach) in the model was not influential enough to significantly modify the results obtained using the isotope signatures of the xylem water sources alone (non-informative approach). Having this agreement between models, we present the results of the water source contribution based on the informative model runs. Results of the non-informative approach are provided in the Supplement.
The model results showed that the intermediate and deep soil water pools (>15 to 120 cm soil depth) were the main sources for the shade trees over the course of the 2014 dry season (91±37 % on average; Fig. 6 and Supplement). Across this period, L. guatemalensis showed on average the highest proportion of water uptake between 30 and 120 cm soil depth (49±26 %), while T. micrantha and I. vera depended strongly on soil water sources between 15 and 30 cm (54±18 % and 67±6 %) (p<0.001). In contrast, the water uptake of coffee plants was mainly sustained by sources from the first 15 cm of soil (94±27 % on average; Fig. 6 and Supplement), having significant differences with all shade tree species (p<0.001).
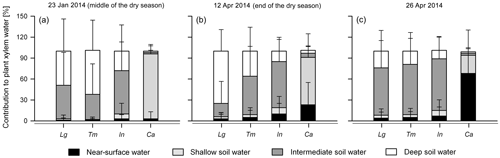
Figure 6MixSIAR Bayesian mixing model results showing the mean likely contribution of each water source to the xylem water of shade canopy trees and coffee shrubs. (a)–(c) show results for the sampling dates of 23 January and 12 and 26 April 2014, respectively, using the informative prior distribution. Lg: L. guatemalensis; Tm: T. micrantha; In: I. vera; and Ca: Coffea arabica. Error bars represent 1 standard deviation of uncertainty.
During the 2017 dry season, the same trend with most water extracted from intermediate and deep soil layers was observed in the shade trees (91±39 % on average; Fig. 7a–c and Supplement). Among sampling dates, differences between tree species only appeared to occur at the end of the dry period (5 April) (p<0.05). Coffee water sources were again restricted to much shallower soil layers (0–5 cm: 53±44 % and 5–15 cm: 42±41 %; Fig. 7a–c and Supplement) compared to shade trees.
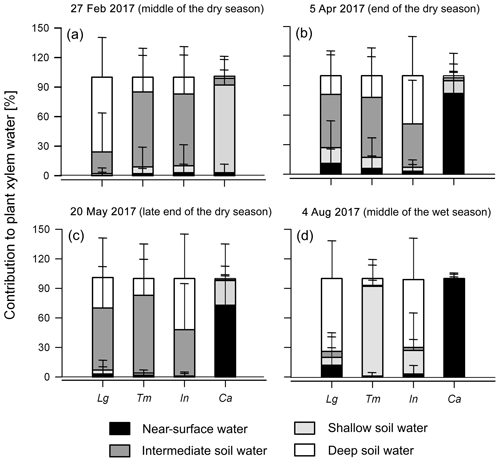
Figure 7MixSIAR Bayesian mixing model results showing the mean likely contribution of each water source to the xylem water of shade canopy trees and coffee shrubs. (a)–(d) show results for the sampling dates of 27 February, 5 April, 20 May and 4 August 2017, respectively, using the informative prior distribution. Lg: L. guatemalensis; Tm: T. micrantha; In: I. vera; and Ca: Coffea arabica. Error bars represent 1 standard deviation of uncertainty.
Overall, we did not find any statistically significant difference among the main plant water sources between the dry periods investigated (p<0.05). Across the individual samplings throughout the two dry seasons, we observed that antecedent precipitation had a stronger effect on the water uptake sources of coffee plants than trees (Fig. 8). For example, when dry antecedent wetness prevailed (API15<5 mm; Fig. 2b), coffee water sources were mainly composed of soil water from >5 to 15 cm depth (91±3 %). Alternatively, when wetter antecedent conditions were present (API15>10 mm), the near-surface soil water layer (58±31 %) was the main contributing source. In contrast, tree water uptake was essentially sustained by deeper soil water sources at low and relatively high antecedent wetness conditions (94±23 % and 87±23 %, respectively) (Fig. 8). Nevertheless, for all species investigated, the relationships between API and the contribution of near-surface soil water sources were not statistically significant (p>0.05).
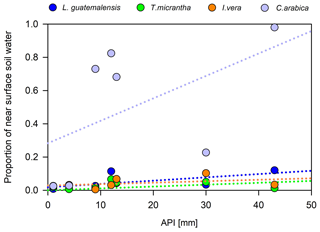
Figure 8Contribution of near-surface soil water to plant uptake at different antecedent precipitation conditions across the 2014 and 2017 dry seasons.
During the 2017 wet season, water source partitioning differed among shade tree species (Fig. 7d and Supplement). During this period, L. guatemalensis and I. vera showed the greatest use of deep soil water (74±37 % and 69±41 %, respectively), while shallower soil water was the main source for T. micrantha (91±23 %), having significant differences with the other tree species (p<0.001). Coffee consistently showed the use of near-surface water sources (98±5 %; Fig. 7d and Supplement), which was significantly different from all shade tree species (p<0.001).
3.6 Fractionation effects on coffee water sources
To evaluate the effects of xylem deuterium fractionation on our results for coffee water source uptake, we compared the relative contribution of each soil water source obtained via the single-isotope (δ2H) mixing model with those obtained via the informative two-isotope mixing model. In general, we observed that the δ2H model consistently estimated a lower contribution of the shallow soil water source and a higher contribution of the near-surface soil water source (Supplement). On average, the reduction in the shallow soil water source ( %) coincided very well with the increase in the near-surface soil water source ( %). These differences were most pronounced for the 2017 dry season samplings (p>0.05; Supplement), during which the differences in δ2H between coffee xylem water and soil water were greatest. However, there were no significant differences between the relative contributions of the intermediate and deep soil water sources estimated by the two models (p>0.05). In summary, the results of the δ2H mixing model suggested an even more pronounced soil water partitioning between coffee and shade tree species than those obtained with the informative two-isotope mixing model.
4.1 Methodological aspects
To our knowledge, the ecohydrological study presented here is one of the first that incorporates biophysical properties as prior information alongside plant water source information from stable isotope (δ18O and δ2H) data into a MixSIAR Bayesian mixing model framework, as a way to improve our understanding of the processes that lead to differences in the depth of plant water uptake. Even though our findings did not change significantly by including or excluding the prior information such as soil macronutrients and root data, exploring plant water source partitioning using these two model approaches provided more confidence in our results. Therefore, we call for more studies that combine soil nutrient and root biomass distribution with plant water source information from δ18O and δ2H data, to explore the additional value of these biophysical parameters elucidating plant–soil interactions in different regions and environments.
In recent years, some plant, soil and/or deep subsurface water source studies that have used stable isotopes have identified isotope variation that could be the result of isotope fractionation processes caused by water molecules interacting with clay surfaces, partially filled pore spaces or salts (Oerter et al., 2014; Oshun et al., 2016; Chen et al., 2016; Lin et al., 2018; Gaj and McDonnell, 2019; Gaj et al., 2017). Our soils were rich in clay content, and according to some studies this type of soil structure can impart isotope fractionation (Meißner et al., 2014; Oerter et al., 2014; Orlowski et al., 2016a; Lin et al., 2018). Thus far, however, these isotope effects have been more evident in clay-rich soils with high cation exchange capacities (CEC ∼ 30 to 70 cmolc kg−1; Oerter et al., 2014; Orlowski et al., 2016b) in combination with low soil water contents (SWC < 20 %; Meißner et al., 2014; Orlowski et al., 2016b). In this respect, the soils in our study area are characterized by low CEC (<21 cmolc kg−1; Table 4). This reflects relatively little interaction between cations adsorbed and clay mineral particles, which indirectly suggests minimal impacts of interlayer water bound in the soil structure (cf. Vidal and Dubacq, 2009). In addition, our soil samples were collected at relatively high SWC across the different sampling periods (∼30 % to 60 %; Fig. 1). As such, we have assumed that the probability of fractionation due to soil properties that may impact water extraction efficiency was very small or completely absent and, therefore, the extracted soil water was the same the plants had access to.
With regard to our plant samples, we specifically observed enrichment in the deuterium composition of the xylem water in the coffee plants in comparison to bulk soil water. It is not surprising that fractionation was evident for δ2H and not δ18O, given the higher fractionation factor of 2H relative to 18O (Rundel et al., 2012). Some possible explanations for this xylem water enrichment could be related to bark evaporation (Ellsworth and Sternberg, 2015) and/or xylem–phloem water exchange (Cernusak et al., 2005), since we did not remove the bark and cambium from our coffee branch samples. On the other hand, like many other crops, coffee plants associate symbiotically with arbuscular mycorrhizal fungi (López-Andrade et al., 2009; Perea Rojas et al., 2019). Studies in our coffee growing region of Veracruz have documented the presence of mycorrhizal structures in coffee roots (Muleta et al., 2008; Arias et al., 2012), which can promote increases in plant water and nutrient uptake (Scheneiger and Jakobsen, 2000; Augé, 2004). Although no research has been carried out yet to test the influence of mycorrhizal fungi on isotope fractionation during coffee root water uptake, this effect could have been present and also responsible for the isotopic mismatch between the coffee xylem water and soil water sources.
We did evaluate the effects of these isotope enrichments in the coffee xylem water on the relative contributions of the coffee water sources using a single-isotope (δ2H) mixing model. Consistently, the model results estimated a higher near-surface water and a lower shallow soil water source contribution in comparison to the dual-isotope informative prior mixing model. In contrast, the estimated proportions of the intermediate and deep soil water sources were similar between models. Thus, the effect of fractionation was translated into a more pronounced spatial separation between the main soil water sources of the coffee plants and shade trees, but our overall results were not different.
4.2 Complementary water use strategy between shade trees and coffee shrubs
Our findings showed that all shade tree species (L. guatemalensis, I. vera and T. micrantha) relied mainly on water sources from deep soil layers (>15 to 120 cm depth), while the use of much shallower water sources (<15 cm) was observed in the coffee (C. arabica var. typica) over the course of the near-normal and more pronounced dry seasons studied. These findings suggest a spatial and temporal partitioning of soil water sources between shade trees and coffee plants during drier periods and water-resource complementary in this coexistence species environment.
Although comparisons of our findings with other traditional shade Arabica coffee plantations are difficult because studies are essentially lacking in this type of agroecosystem, there are a handful of other investigations carried out in shade coffee monospecific plantations in the humid tropics in which complementary rather than competitive water use strategies prevailed. For example, Cannavo et al. (2011) compared the water use and soil water availability of an unshaded coffee vs. a shaded monoculture (Inga densiflora) coffee plantation in Costa Rica, both 7–8 years old, using soil moisture measurements and water balance calculations. Their results showed that soil water content in the deeper soil layers (>120 cm depth) was lower in the shaded coffee system than in the sun-grown coffee system, while water content in the shallower layers was similar. This suggested that associated shade trees preferentially used water from deeper soil horizons, providing some evidence of complementarity water use between coffee plants and native Inga trees during the dry season. However, the authors acknowledged that they were unable to separate roots of coffee from those of trees in the soil profiles, so they could not be certain whether trees were the only individuals extracting water from deeper sources. In this respect, our study showed that there was always a mixture in water uptake from different sources (soil group depths), but a separation between the main sources of water for shade trees and coffee shrubs clearly prevailed.
Other investigations in Costa Rica have examined the below-ground resource competition of Arabica coffee in association with fast-growing timber species using data of plant growth, root distribution and density, and soil moisture and nutrient patterns. For example, the study of Schaller et al. (2003) carried out in a commercial (Eucalyptus deplupta) shade coffee plantation where soils are highly fertilized showed that coffee had a relatively even root distribution along the first 40 cm of soil depth, with a higher root density in the proximity of the coffee rows. Conversely, the root system of E. deplupta was much shallower, with most roots concentrated in the upper 10 cm of the soil. In this case, the tree root density was found to be highest in the alleys between the coffee rows. The authors explained that the apparent complementary resource exploitation of this tree–crop system was mainly attributed to high availability of soil resources and the high competitiveness of the coffee limiting the expansion of tree roots (cf. Lehmann, 2003). Although in our study we did not determine the depth distribution of coffee and tree roots, our findings showed that all shade tree species were tapping water from deeper soil layers than coffee, suggesting that trees are deep rooted and able to explore larger soil volumes, causing little competition with coffee.
In Nicaragua, Padovan et al. (2015) compared the root distribution, soil moisture, transpiration and leaf water potential patterns in a sun-grown coffee system and an agroforestry of coffee planted with two timber trees (deciduous Tabebuia rosea and evergreen Simarouba glauca). Their findings showed that coffee roots were more abundant than tree roots and mainly concentrated in the shallower soil layers (0–80 cm depth). Most roots of both tree species were observed in deeper layers (>100 cm), suggesting a clear niche differentiation with coffee. During the 3-year study period, volumetric water content along a 2 m soil profile was higher in the sun-grown coffee than in the shaded coffee, which was explained by greater soil water uptake from trees below the crop rooting zone (Padovan et al., 2015). Moreover, coffee shrubs in the shaded plantation were more water stressed (i.e., lowest midday leaf water potentials) during the pronounced dry season studied (Padovan et al., 2018). Their results suggest that despite the clear hydrological niche segregation, competition between coffee and shade trees may occur if the dry season is long and severe enough.
Our findings also showed that during the wet season coffee plants substantially increased the use of near-surface water (+56 %) in comparison to the dry season, while all shade trees also extended their water acquisition to much shallower soil water pools (+19 %). This is largely explained by the increases in soil moisture in the first 30 cm depth due to frequent rainfall inputs that characterize the wet season in our study area. This also suggests that coffee had a higher root activity in the top soil layers during the wet season in comparison to the dry season, as has been documented in other studies (Huxley et al., 1974). Regarding the shade trees, we observed that T. micrantha showed the greatest response to wetter conditions by drawing most water from the first 15 cm of soil (92 %), whereas this was much less evident in L. guatemalensis (21 %) and I. vera (27 %). Although we did not determine the vertical distribution of roots for each of the shade tree species studied, these findings suggest that T. micrantha has a shallower rooting system than the other tree species. The fact that the T. micrantha trees were more recently planted (i.e., younger with a less developed root system) than the L. guatemalensis and I. vera trees supports this idea. On the other hand, the high temperature and rainfall that characterize the wet season at our study site may favor rapid mineralization of nutrients and their subsequent leaching to deeper soil layers (i.e., potassium, calcium and magnesium; Table 4). Hence, for the larger trees studied (L. guatemalensis), the availability of water and nutrients at deeper depths could have been an important resource for plant growth in this period, partly explaining the lower activity of their shallower roots. Despite the changes and the higher variability in depth of water uptake observed among canopy trees and coffee shrubs, a complementary use of soil water prevailed during the wet season. Future work should be focused on the distribution and dynamics of tree and crop roots and their seasonal variation in relation to the availability of nutrients and water in the soil. Also, it would be desirable to relate these dynamics to crop and shade tree phenology to elucidate temporal synergistic or competitive water requirements.
4.3 The role of antecedent wetness in coffee water uptake
Despite the relatively small sample size, our study showed that antecedent wetness strongly influenced the water uptake patterns of coffee plants (cf. Huxley et al., 1974). We found that under relatively wet antecedent conditions prevailing after small rainfall events during the dry season, coffee substantially increased the use of near-surface soil water sources, possibly as an opportunistic strategy to overcome the soil water deficits in this period and take advantage of their much shallower rooting system compared to trees. Conversely, tree water uptake was mainly sourced by deeper soil water layers showing less sensitiveness to higher antecedent wetness. In this respect there are no comparative studies in shade coffee agroecosystems evaluating the functional response of plant water uptake over a range of antecedent wetness. Nevertheless, plant and soil water interactions under dry and relatively wet conditions have been examined in other types of agroforestry systems. For example, in the study of Gao et al. (2018) carried out in a semiarid region in China, the authors evaluated the seasonal variations in water use of jujube (Ziziphus jujuba) trees planted with annual (Brassica napus) and perennial (Hemerocallis fulva) crops. Using stable isotope techniques and Bayesian mixing modeling, their results showed that jujube trees generally tapped water (>58 %) from deep soil layers (60–200 cm depth) at low antecedent wetness, while both B. napus and H. fulva crops primarily extracted water (>65 %) from intermediate (20–60 cm) and shallow (0–20 cm) soil layers. This exhibits a complementary water use strategy between trees and crops. However, at higher antecedent wetness both the jujube trees and crops extracted most water from the first 0 to 60 cm of soil depth (>65 %). This indicated that both species exhibited an opportunistic strategy for accessing resources at shallower soil depths. In this case, contrary to our findings, tree roots rather than crop roots showed the stronger capacity to switch rapidly from deep to shallow sources in response to increased soil water availability.
4.4 Implications and future directions
The consistent complementarity in plant water use strategies observed under different hydrometeorological conditions in the coffee plantation studied provides support to the central tenet of agroforestry systems (Cannel et al., 1996). Based on our findings, L. guatemalensis, I. vera and T. micrantha provide good choices for coffee shade trees due to their complementarity in soil water use. Since these tree species obtained their water from deeper soil layers than the coffee, this could mean that they utilize nutrients leaching beyond the reach of the coffee plants and so contribute to improved nutrient cycling and increased overall productivity of the system (van Noordwijk et al., 2015).
Nevertheless, the current outcome may change given the new coffee management practices that consist in replacing traditional coffee varieties (e.g., C. arabica var. typica) with others (C. arabica var. costa rica; C. canephora) that may exhibit deeper root systems and perhaps different water (and nutrient) uptake strategies, in response to prevalent diseases such as leaf rust or root nematodes. Therefore, future research should be focused on evaluating the water source partitioning of traditional vs. new coffee disease-resistant varieties and their relation to shade tree water use. In this respect, there are further questions with regard to strategic use of shade tree species, whereby fast-growing species might be more (commercially) productive but also more competitive. Some evidence from elsewhere has shown that such management practices do not necessarily increase competition and may even enhance the water use efficiency as part of drought-avoidance mechanisms. For example, in Southeast China, Wu et al. (2016) used δ2H and δ18O stable isotope methods to examine the seasonal water use of a fast-growing rubber tree species (Hevea brasilensis) planted with Arabica coffee. Their findings showed that rubber trees mostly accessed water from intermediate (15–50 cm depth) and deep soil layers (50–110 cm); meanwhile, coffee mostly tapped water from the topsoil (<15 cm). Additionally, rubber trees showed strong root plasticity in soil water uptake, avoiding competition with coffee during the rainy and relatively dry seasons. However, more research is needed since these results depend largely on tree–crop species combinations and local climatic and soil conditions.
In addition to effects of changing management practices, climate warming may induce changes in plant transpiration throughout the year (e.g., Karmalkar et al., 2011). In our study, we used a water stable isotope approach along with root and soil macronutrient data to estimate the relative contribution of the plant water sources. However, for a more complete assessment of the plant and soil interactions, seasonal plant water fluxes need to be quantified. Our results so far have made the first steps towards serving coffee producers to make better decisions on sustainable coffee and water management as well as providing new insights into water resources in general, which are urgently required for implementing efficient and equitable management programs in humid tropical environments (Hamel et al., 2018). However, future work should be focused on water use of individual trees and coffee shrubs using ecophysiological and hydrological techniques in order to know how much water is used from the different soil water pools.
This study provides the first baseline information on plant water sources for a traditional shade coffee plantation in the humid tropics. Our results showed that coffee water uptake was mainly sustained from shallow soil sources (<15 cm depth), while all shade trees relied on water sources from deeper soil layers (>15 to 120 cm depth). This complementary strategy in soil water use between crops and trees was consistent over the course of the near-normal and more pronounced dry seasons investigated. Across these same periods, we observed that antecedent precipitation had a strong influence in coffee plants, increasing their water uptake to near-surface soil water sources as an opportunistic strategy to overcome the reduced water availability. In the wet season, coffee plants substantially increased the use of near-surface water (<5 cm depth), whereas shade trees expanded their water acquisition to the first 15 cm of soil depth. Overall, a greater soil water partitioning prevailed among tree and coffee species when higher soil moisture conditions were present. Nevertheless, despite such variability in plant–soil water interactions across seasons, a clear spatial segregation of the main water source prevailed between shade trees and coffee plants during the rainy and dry periods investigated.
Data can be accessed at https://doi.org/10.5063/F1MS3R3J (Muñoz-Villers et al., 2020).
The supplement related to this article is available online at: https://doi.org/10.5194/hess-24-1649-2020-supplement.
LEMV designed the experiment. LEMV, MSAB and FH collected the field data. MSAB performed all the Bayesian mixing model analysis. JG contributed in the data analysis. LEMV prepared the first draft of the manuscript. FH, MSAB and JG edited and commented on the manuscript several times, and TED carried out the final revision. Later, all the co-authors contributed with revisions.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Water, isotope and solute fluxes in the soil–plant–atmosphere interface: investigations from the canopy to the root zone”. It is not associated with a conference.
We would like to thank Raul Monge and Daniel Tejeda for their permission to conduct this research in La Orduña coffee plantation. We also thank Melissa López-Portillo for performing the cryogenic extractions and Stefania Mambelli and Wembo Yang for analyzing the water isotope samples at UC-Berkeley, USA. Angel Zaragoza, Erika Mendoza, Alitzel Guzmán and Carlos Alcocer are thanked for their assistance in the sampling campaigns. The Instituto de Ecología, A. C. (INECOL) is thanked for the laboratory facilities to carry out the root separation and oven-dry weights. We also thanked Adriana Hernández and Esperanza Huerta for helping in the root separation process. Data analysis and partial writing of the manuscript were performed during LEMV's sabbatical leave (March–July 2018) hosted by Josie Geris at the University of Aberdeen, UK, and granted by the Programa de Apoyos para la Superación de Personal Académico (PASPA) of the UNAM. Lastly, we appreciate the valuable comments of reviewers Adriá Barbeta, Matthias Beyer and Daniele Penna and editor Matthias Sprenger that helped to improve an earlier version of the manuscript.
This research has been supported by the PAPIIT-UNAM (Mexico) (grant nos. IB100313 and IB100113), the CONACyT (Mexico) (grant no. 187646), the National Science Foundation (US) (grant no. 1313804), and the Scottish Funding Council (UK) (grant no. SF10192).
This paper was edited by Matthias Sprenger and reviewed by Daniele Penna, Adrià Barbeta, and Matthias Beyer.
Arias, R. M., Heredia, G., Sosa, V., and Fuentes-Ramírez, L. E.: Diversity and abundance of arbuscular mycorrhizal fungi spores under different coffee production systems and in a tropical montane cloud forest patch in Veracruz, Mexico, Agroforest. Syst., 85, 179–193, https://doi.org/10.1007/s10457-011-9414-3, 2012.
Augé, R. M.: Arbuscular mycorrhizae and soil/plant water relations, Can. J. Soil Sci., 84, 373–381, https://doi.org/10.4141/S04-002, 2004.
Baca, M., Läderach, P., Haggar, J., Schroth, G., and Ovalle, O.: An integrated framework for assessing vulnerability to climate change and developing adaptation strategies for coffee growing families in Mesoamerica, PLoS ONE, 9, e88463, https://doi.org/10.1371/journal.pone.0088463, 2014.
Báez, A. P., Padilla, H., Cervantes, J., Pereyra, D., and Belmont, R.: Rainwater chemistry at the eastern flanks of the Sierra Madre Oriental, Veracruz, Mexico, J. Geophys. Res.-Atmos., 102, 23329–23336, 1997.
Baker, P. S. and Haggar, J.: Global warming: the impact on global coffee, in: SCAA Conference Handout, Long Beach, USA, 2007.
Barbeta, A., Mejia-Chang, M., Ogaya, R., Voltas, J., Dawson, T. E., and Peñuelas, J.: The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest, Global Change Biol., 21, 1213–1225, https://doi.org/10.1111/gcb.12785, 2015.
Barbeta, A., Jones, S. M., Clavé, L., Wingate, L., Gimeno, T. E., Fréjaville, B., Wohl, S., and Ogée, J.: Unexplained hydrogen isotope offsets complicate the identification and quantification of tree water sources in a riparian forest, Hydrol. Earth Syst. Sci., 23, 2129–2146, https://doi.org/10.5194/hess-23-2129-2019, 2019.
Beer, J., Muschler, R., Kass, D., and Somarriba, E.: Shade management in coffee and cacao plantations, Agroforest. Syst., 38, 139–164, https://doi.org/10.1023/A:1005956528316, 1998.
Beyer, M., Hamutoko, J. T., Wanke, H., Gaja, M., and Koeniger, P.: Examination of deep root water uptake using anomalies of soil water stable isotopes, depth-controlled isotopic labeling and mixing models, J. Hydrol., 566, 122–136, https://doi.org/10.1016/j.jhydrol.2018.08.060, 2018.
Bray, R. H. and Kurtz, L.: Determination of total, organic, and available forms of phosphorus in soils, Soil Sci., 59, 39–46, 1945.
Bunn, C., Läderach, P., Ovalle Rivera, O., and Kirschke, D.: A bitter cup: climate change profile of global production of Arabica and Robusta coffee, Climatic Change, 129, 89–101, https://doi.org/10.1007/s10584-014-1306-x, 2015.
Cannavo, P., Sansoulet, J., Harmand, J.-M., Siles, P., Dreyer, E., and Vaast, P.: Agroforestry associating coffee and Inga densiflora results complementarity for water uptake and decreases deep drainage in Costa Rica, Agr. Ecosyst. Environ., 140, 1–13, https://doi.org/10.1016/J.AGEE.2010.11.005, 2011.
Cannell, M. G. R., Van Noordwijk, M., and Ong, C. K.: The central agroforestry hypothesis: the trees must acquire resources that the crop would not otherwise acquire, Agroforest. Syst., 34, 27–31, https://doi.org/10.1007/BF00129630, 1996.
Cernusak, L. A., Farquhar, G. D., and Pate, J. S.: Environmental and physiological controls over oxygen and carbon isotope composition of Tasmanian blue gum, Eucalyptus globulus, Tree Physiol., 25, 129–146, https://doi.org/10.1093/treephys/25.2.129, 2005.
Chen, G., Auerswald, K., and Schnyder, H.: 2H and 18O depletion of water close to organic surfaces, Biogeosciences, 13, 3175–3186, https://doi.org/10.5194/bg-13-3175-2016, 2016.
Dansgaard, W.: Stable isotopes in precipitation, Tellus, 16, 436–468, https://doi.org/10.3402/tellusa.v16i4.8993, 1964.
Dawson, T. E., Mambelli, S., Plamboeck, A. H., Templer, P. H., and Tu, K. P.: Stable Isotopes in Plant Ecology, Annu. Rev. Ecol. Syst., 33, 507–559, https://doi.org/10.1146/annurev.ecolsys.33.020602.095451, 2002.
Digital Globe: QuickBird Satellite Image of central Veracruz, available at: https://gbdxdocs.digitalglobe.com/docs/quickbird (last access: 30 January 2014), 2010.
Ellsworth, P. Z. and Sternberg, L. S.: Seasonal water use by deciduous and evergreen woody species in a scrub community is based on water availability and root distribution, Ecohydrology, 8, 538–551, https://doi.org/10.1002/eco.1523, 2015.
Escamilla, P. E., Ruiz, R. O., Díaz, P. G., Landeros, S. C., Platas, R. D. E., Zamarripa, C. A., and González, H. V. A.: El agroecosistema café orgánico en México, Manejo Integrado de Plagas y Agroecología, 76, 5–16, 2005.
Evaristo, J., McDonnell, J. J., and Clemens, J.: Plant source water apportionment using stable isotopes: A comparison of simple linear, two-compartment mixing model approaches, Hydrol. Process., 31, 3750–3758, https://doi.org/10.1002/hyp.11233, 2017.
Gaj, M. and McDonnell, J. J.: Possible soil tension controls on the isotopic equilibrium fractionation factor for evaporation from soil, Hydrol. Process., 33, 1629–1634, https://doi.org/10.1002/hyp.13418, 2019.
Gaj, M., Kaufhold, S., Koeniger, P., Beyer, M., Weiler, M., and Himmelsbach, T.: Mineral mediated isotope fractionation of soil water, Rapid Commun. Mass Spectrom., 31, 269–280, https://doi.org/10.1002/rcm.7787, 2017.
Gao, X., Liud, Z., Zhao, X., Ling, Q., Huo, G., and Wu, P.: Extreme natural drought enhances interspecific facilitation in semiarid agroforestry systems, Agr. Forest. Meteorol., 265, 444–453, https://doi.org/10.1016/j.agee.2018.07.001, 2018.
García, E.: Modificaciones al sistema de clasificación climática de Köppen, Offset Larios, México, D. F., México, 217 pp., 1988.
Gelman, A., Carlin, J. B., Stern, H. S., Dunson, D. B., Vehtari, A., and Rubin, D. B.: Bayesian Data Analysis, Taylor and Francis Group, London, UK, 2014.
Giorgi, F.: Climate change hot-spots, Geophys. Res. Lett., 33, L08707, https://doi.org/10.1029/2006GL025734, 2006.
Greenberg, R., Bichier, P., and Sterling, J.: Bird populations in rustic and planted shade coffee plantations of eastern Chiapas, Mexico, Biotropica, 29, 501–514, https://doi.org/10.1111/j.1744-7429.1997.tb00044.x, 1997.
Hamel, P., Riveros-Iregui, D., Ballari, D., Browning, T., Célleri, R., Chandler, D., Chun, K. P., Destouni, G., Jacobs, S., Jasechko, S., Johnson, M., Krishnaswamy, J., Poca, M., Vieira Pompeu, P., and Rocha, H.: Watershed services in the humid tropics: Opportunities from recent advances in ecohydrology, Ecohydrology, 11, e1921, https://doi.org/10.1002/eco.1921, 2018.
Hernández-Martínez, G., Manson, R. H., and Contreras-Hernández, A.: Quantitative classification of coffee agroecosystems spanning a range of production intensities in central Veracruz, Mexico, Agr. Ecosyst. Environ., 134, 89–98, https://doi.org/10.1016/j.agee.2009.05.020, 2009.
Hernández-Martínez, G., Escamilla-Femat, S., Velázquez-Premio, T., and Martínez-Marín, J. L.: Análisis de la cadena de suministro del café en el centro de Veracruz: situación actual, retos y oportunidades, in: Cafeticultura en la zona centro del estado de Veracruz: diagnóstico, productividad y servicios ambientales, edited by: López-Morgado, R., Sosa-Fernández, V., Díaz-Padilla, G., and Contreras-Hernández, H. A., INIFAP, México, 8–36, 2013.
Holwerda, F., Bruijnzeel, L. A., Barradas, V. L., and Cervantes, J.: The water and energy exchange of a shaded coffee plantation in the lower montane cloud forest zone of central Veracruz, Mexico, Agr. Forest. Meteorol., 172, 1–13, https://doi.org/10.1016/j.agrformet.2012.12.015, 2013.
Holwerda, F., Alvarado-Barrientos, M. S., and González-Martínez, T. M.: Surface energy exchange in a tropical montane cloud forest environment: Flux partitioning, and seasonal and land cover-related variations, Agr. Forest. Meteorol., 228–229, 13–28, https://doi.org/10.1016/j.agrformet.2016.06.011, 2016.
Huxley, P. A., Patel, R. Z., Kabaara, A. M., and Mitchell, H. W.: Tracer studies with 32P on the distribution of functional roots of Arabica coffee in Kenya, Ann. Appl. Biol., 77, 159–180, https://doi.org/10.1111/j.1744-7348.1974.tb06883.x, 1974.
Jha, S., Bacon, C. M., Philpott, S. M., Méndez, V. E., Laederach, P., and Rice, R. A.: Shade coffee: Update on a disappearing refuge for biodiversity, BioScience, 64, 416–428, https://doi.org/10.1093/biosci/biu038, 2014.
Karmalkar, A. V., Bradley, R. S., and Diaz, H. F.: Climate change in Central America and Mexico: regional climate model validation and climate change projections, Clim. Dynam., 37, 605–629, https://doi.org/10.1007/s00382-011-1099-9, 2011.
Kellermann, J. L., Johnson, M. D., Stercho, A. M., and Hackett, S. C.: Ecological and economic services provided by birds on Jamaican blue mountain coffee farms, Conserv. Biol., 22, 1177–1185, https://doi.org/10.1111/j.1523-1739.2008.00968.x, 2008.
Lehmann, J.: Subsoil root activity in tree-based cropping systems, Plant Soil, 255, 319–321, 2003.
Lin, Y., Horita, J., and Abe, O.: Adsorption isotope effects of water on mesoporous silica and alumina with implications for the land-vegetation-atmosphere system, Geochim. Cosmochim. Ac., 223, 520–536, https://doi.org/10.1016/j.gca.2017.12.021, 2018.
López Andrade, S. A., Mazzafera, P., Schiavinato, M. A., and Silveira, P. D.: Arbuscular mycorrhizal association in coffee, J. Agric. Sci., 147, 105–115, https://doi.org/10.1017/S0021859608008344, 2009.
Marchal, J. and Palma, R.: Análisis gráfico de un espacio regional: Veracruz, INIREB/ORSTOM, México, Xalapa, Veracruz, 220 pp., 1985.
Mas, A. H. and Dietsch, T. V.: Linking shade coffee certification to biodiversity conservation: butterflies and birds in Chiapas, Mexico, Ecol. Appl., 14, 642–654, https://doi.org/10.1890/02-5225, 2004.
Meißner, M., Köhler, M., Schwendenmann, L., Hölscher, D., and Dyckmans, J.: Soil water uptake by trees using water stable isotopes (δ2H and δ18O) – a method test regarding soil moisture, texture and carbonate, Plant Soil, 376, 327–335, https://doi.org/10.1007/s11104-013-1970-z, 2014.
Moguel, P. and Toledo, V. M.: Biodiversity conservation in traditional coffee systems of Mexico, Conserv. Biol., 13, 11–21, https://doi.org/10.1046/j.1523-1739.1999.97153.x, 1999.
Monteith, J. L., Ong, C. K., and Corlett, J. E.: Microclimatic interactions in agroforestry systems, Forest. Ecol. Manage., 45, 31–44, https://doi.org/10.1016/0378-1127(91)90204-9, 1991.
Moore, J. W. and Semmens, B. X.: Incorporating uncertainty and prior information into stable isotope mixing models, Ecol. Lett., 11, 470–480, https://doi.org/10.1111/j.1461-0248.2008.01163.x, 2008.
Muleta, D., Assefa, F., Nemomissa, S., and Granhall, U.: Distribution of arbuscular mycorrhizal fungi spores in soils in smallholder agroforestry and monocultural coffee systems in southwestern Ethiopia, Biol. Fert. Soils, 44, 653–659, https://doi.org/10.1007/s00374-007-0261-3, 2008.
Muñoz-Villers, L. E., Holwerda, F., Alvarado-Barrientos, M. S., Geissert, D. R., and Dawson, T. E.: Reduced dry season transpiration is coupled with shallow soil water use in tropical montane forest trees, Oecologia, 188, 303–317, https://doi.org/10.1007/s00442-018-4209-0, 2018.
Muñoz-Villers, L. E., Geris, J., Alvarado Barrientos, M. S., Holwerda, F., and Dawson, T.: La Orduña Shade Coffee Plantation. Mexico, Hydrometeorological and Water Stable Isotope Data, Knowledge Network for Biocomplexity, https://doi.org/10.5063/F1MS3R3J, 2020.
Nath, C., Pélissier, R., Ramesh, B., and García, C.: Promoting native trees in shade coffee plantations of southern India: comparison of growth rates with the exotic Grevillea robusta, Agroforest. Syst., 83, 107–119, https://doi.org/10.1007/s10457-011-9401-8, 2011.
Oerter, E., Finstad, K., Schaefer, J., Goldsmith, G. R., Dawson, T., and Amundson, R.: Oxygen isotope fractionation effects in soil water via interaction with cations (Mg, Ca, K, Na) adsorbed to phyllosilicate clay minerals, J. Hydrol., 515, 1–9, https://doi.org/10.1016/j.jhydrol.2014.04.029, 2014.
Orlowski, N., Breuer, L., and Mcdonnell, J. J.: Critical issues with cryogenic extraction of soil water for stable isotope analysis, Ecohydrology, 9, 3–10, https://doi.org/10.1002/eco.1722, 2016a.
Orlowski, N., Pratt, D. L., and McDonnell, J. J.: Intercomparison of soil pore water extraction methods for stable isotope analysis, Hydrol. Process., 30, 3434–3449, https://doi.org/10.1002/hyp.10870, 2016b.
Oshun, J., Dietrich, W. E., Dawson, T. E., and Fung, I.: Dynamic, structured heterogeneity of water isotopes inside hillslopes, Water Resour. Res., 52, 164–189, https://doi.org/10.1002/2015WR017485, 2016.
Padovan, M. P., Cortez, V. J., Navarrete, L. F., Navarrete, E. D., Deffner, A. C., Centeno, L. G., Munguía, R., Barrios, M., Vílchez-Mendoza, J. S., Vega-Jarquín, C., Costa, A. N., Brook, R. M., and Rapidel, B.: Root distribution and water use in coffee shaded with Tabebuia rosea Bertol. and Simarouba glauca DC. compared to full sun coffee in sub-optimal environmental conditions, Agroforest. Syst., 89, 857–868, https://doi.org/10.1007/s10457-015-9820-z, 2015.
Padovan, M. P., Brook, R. M., Barrios, M., Cruz-Castillo, J. B., Vilchez-Mendoza, S. J., Costa, A. N., and Rapidel, B.: Water loss by transpiration and soil evaporation in coffee shaded by Tabebuia Rosea Bertol. and Simarouba Glauca Dc. Compared to unshaded coffee in sub-optimal environmental conditions, Agr. Forest. Meteorol., 248, 1–14, https://doi.org/10.1016/j.agrformet.2017.08.036, 2018.
Parnell, A. C., Inger, R., Bearhop, S., and Jackson, A. L.: Source partitioning using stable isotopes: Coping with too much variation, PloS One, 5, e9672, https://doi.org/10.1371/journal.pone.0009672, 2010.
Penna, D., Hopp, L., Scandellari, F., Allen, S. T., Benettin, P., Beyer, M., Geris, J., Klaus, J., Marshall, J. D., Schwendenmann, L., Volkmann, T. H. M., von Freyberg, J., Amin, A., Ceperley, N., Engel, M., Frentress, J., Giambastiani, Y., McDonnell, J. J., Zuecco, G., Llorens, P., Siegwolf, R. T. W., Dawson, T. E.n and Kirchner, J. W.: Ideas and perspectives: Tracing terrestrial ecosystem water fluxes using hydrogen and oxygen stable isotopes – challenges and opportunities from an interdisciplinary perspective, Biogeosciences, 15, 6399–6415, https://doi.org/10.5194/bg-15-6399-2018, 2018.
Perea Rojas, Y., Arias, R. M., Medel Ortiz, R., Trejo Aguilar, D., Heredia, G., and Rodríguez, Y.: Effects of native arbuscular mycorrhizal and phosphate-solubilizing fungi on coffee plants, Agroforest. Syst., 93, 961–972, https://doi.org/10.1007/s10457-018-0190-1 2019.
Perfecto, I., Mas, A., Dietsch, T., and Vandermeer, J.: Conservation of biodiversity in coffee agroecosystems: a tri-taxa comparison in southern Mexico, Biodivers. Conserv., 12, 1239–1252, https://doi.org/10.1023/A:1023039921916, 2002.
Perfecto, I., Armbrecht, I., Philpott, S. M., Soto-Pinto, L., and Dietsch, T. M.: Shaded coffee and the stability of rainforest margins in northern Latin America, in: The Stability of Tropical Rainforest Margins, Linking Ecological, Economic and Social Constraints of Land Use and Conservation, Environmental Science Series, edited by: Tscharntke, T., Leuschner, C., Zeller, M., Guhadja, E., and Bidin, A., Springer Verlag, Berlin, 227–264, 2007.
R Development Core Team: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-900051-07-0, available at: http://www.R-proje ct.org, last access: 30 April 2016.
Renard, M. C.: The Mexican coffee crisis, Lat. Am. Perspect., 37, 21–23, https://doi.org/10.1177/0094582X09356956, 2010.
Rice, R. A.: Coffee in the crosshairs of climate change: agroforestry as abates, Agroecol. Sust. Food, 42, 1058–1076, https://doi.org/10.1080/21683565.2018.1476428, 2018.
Rodríguez, S. R., Morales-Barrera, W., Layer, P., and González-Mercado, E.: A quaternary monogenetic volcanic field in the Xalapa region, Eastern Trans-Mexican volcanic belt: Geology, distribution and morphology of the volcanic vents, J. Volcanol. Geoth. Res., 197, 149–166, https://doi.org/10.1016/j.jvolgeores.2009.08.003, 2010.
Rundel, P. W., Ehleringer, J. R., and Nagy, K. A.: Stable isotopes in ecological research, in: Ecological Studies 68, Springer-Verlag, New York, 525 pp., 2012.
Schaller, M., Schroth, G., Beer, J., and Jiménez, F.: Species and site characteristics that permit the association of fast-growing trees with crops: the case of Eucalyptus deglupta as coffee shade in Costa Rica, Forest. Ecol. Manage., 175, 205–215, https://doi.org/10.1016/S0378-1127(02)00079-8, 2003.
Scheneiger, P. and Jakobsen, I.: Laboratory and field methods for measurement of hyphal uptake of nutrients in soil, Plant Soil, 226, 237–244, https://doi.org/10.1023/A:1026578018230, 2000.
Schroth, G., Rodrigues, M. R. L., and D'Angelo, S. A.: Spatial patterns of nitrogen mineralization, fertilizer distribution and roots explain nitrate leaching from mature Amazonian oil palm plantation, Soil Use Manage., 16, 222–229, https://doi.org/10.1111/j.1475-2743.2000.tb00197.x, 2000.
Schroth, G., Laderach, P., Dempewolf, J., Philpott, S., Haggar, J., Eakin, H., Castillejos, T., Garcia Moreno, J., Soto Pinto, L., Hernandez, R., Eitzinger, A., and Ramirez-Villegas, J.: Towards a climate change adaptation strategy for coffee communities and ecosystems in the Sierra Madre de Chiapas, Mexico, Mitig. Adapt. Strat. Gl., 14, 605–625, https://doi.org/10.1007/s11027-009-9186-5, 2009.
SMN: Climatic Normals Published on the Website of the National Weather Service of Mexico, available at: http://smn.cna.gob.mx/ (last access: 16 April 2018), 2014.
Stock, B. C. and Semmens, B. X.: MixSIAR GUI user manual, Version 3.1, available at: https://githu b.com/brian stock /MixSI AR/, last access: 14 May 2017.
Tscharntke, T., Clough, Y., Bhagwat, S. A., Buchori, D., Faust, H., Hertel, D., Hölscher, D., Juhrbandt, J., Kessler, M., Perfecto, I., and Scherber, C.: Multifunctional shade-tree management in tropical agroforestry landscapes – a review, J. Appl. Ecol., 48, 619–629, https://doi.org/10.1111/j.1365-2664.2010.01939.x, 2011.
USDA: PSD Online – Home, in: Production, Supply and Distribution Online, available at: http://www.fas.usda.gov/psdonline/, last access: 14 May 2018.
Vaast, P., Harmand, J.-M., Rapidel, B., Jagoret, P., and Deheuvels, O.: Coffee and Cocoa Production in Agroforestry-A Climate-Smart Agriculture Model, in: Climate Change and Agriculture Worldwide, edited by: Torquebiau, E., Springer, Dordrecht, the Netherlands, 209–224, https://doi.org/10.1007/978-94-017-7462-8_16, 2016.
van Kanten, R., Schroth, G., Beer, J., and Jiménez, F.: Fine-root dynamics of coffee in association with two shade trees in Costa Rica, Agroforest. Syst., 63, 247–261, https://doi.org/10.1007/s10457-005-4163-9, 2005.
van Noordwijk, M., Lawson, G., Hairiah, K., and Wilson, J.: Root distribution of trees and crops: competition and/or complementarity, in: Tree-crop interactions: agroforestry in a changing climate, edited by: Ong, C. K., Black, C. R., and Wilson, J., CAB International, Wallingford, UK, 1–44, 2015.
Van Reeuwijk, L. P.: Procedures for soil analysis. International Soil Reference and Information Centre, ISRIC, FAO, Wageningen, 2002.
Vidal, O. and Dubacq, B.: Thermodynamic modelling of clay dehydration, stability and compositional evolution with temperature, pressure and H2O activity, Geochim. Cosmochim., Ac., 73, 6544–6564, https://doi.org/10.1016/j.gca.2009.07.035, 2009.
Viessman, W., Lewis, G. L., and Knapp, J. W.: Introduction to Hydrology, Harper Collins, New York, 612 pp., 1989.
Villers, L., Arizpe, N., Orellana, R., Conde, C., and Hernández, J.: Impactos del cambio climático en la floración y desarrollo del fruto del café en Veracruz, México, Interciencia, 34, 322–329, 2009.
West, A. G., Patrickson, S. J., and Ehleringer, J. R.: Water extraction times for plant and soil materials used in stable isotope analysis, Rapid Commun. Mass Spectrom., 20, 1317–1321, https://doi.org/10.1002/rcm.2456, 2006.
Wu, J., Liu, W., and Chen, C.: Can intercropping with the world's three major beverage plants help improve the water use of rubber trees? J. Appl. Ecol., 53, 1787–1799, https://doi.org/10.1111/1365-2664.12730, 2016.