the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Technical note: A new laboratory approach to extract soil water for stable isotope analysis from large soil samples
Jan Haidl
Ondrej Gebousky
Kristyna Falatkova
Vaclav Sipek
Martin Sanda
Natalie Orlowski
Lukas Vlcek
A correct soil water extraction represents an initial step in stable water isotope analysis. With this aim, we present a new soil water extraction method based on the principle of complete evaporation and condensation of the soil water in a closed circuit. The proposed device has four extraction slots and can be used up to two times a day. Owing to its simple design, there is no need for any chemicals, gases, or high-pressure or high-temperature regimes. The experimental tests proved that the extraction itself does not cause any major isotope fractionation effects leading to erroneous results. Extraction of pure-water samples shifts the isotope composition by 0.04 ± 0.06 ‰ and 0.06 ± 0.35 ‰ for δ18O and δ2H, respectively. Soil water extraction tests were conducted for five distinct soil types (loamy sand, sandy loam, sandy clay, silt loam, and clay) using 40–150 g of pre-oven-dried soil, which was subsequently rehydrated to 10 % and 20 % water content. The shift in the isotopic composition of these tests ranged between −0.04 ‰ and 0.07 ‰ for δ18O and 0.4 ‰ and 1.3 ‰ for δ2H, with the standard deviations of ± (0.08–0.25) ‰ and ± (0.34–0.58) ‰ for δ18O and δ2H, respectively. The results exhibit high accuracy, which makes this method suitable for high-precision studies where unambiguous determination of the water origin is required.
- Article
(1349 KB) - Full-text XML
- BibTeX
- EndNote
Measurements of soil water isotopic composition (2H and 18O) provide a description of soil water movement and mixing processes in the vadose zone (Stumpp et al., 2018). In some cases, different trends in soil water sample characterization without the application of an exact isotopic composition method (tracer experiments to prove interconnection) give sufficient information about sample dissimilarity. However, for characterizing the transport processes and residence times, accurate evaluation of the sample origin, soil water dynamic modelling, or inter-laboratory comparison, the exact values of the isotopic composition are indispensable. This justifies the emphasis placed on correct soil water extraction. Unlike liquid-water samples of precipitation, snow cover, stream water, or groundwater, where the isotopic compositions are easily accessible, the extraction of matrix-bound or tightly bound soil water is challenging from the viewpoint of exact determination of isotopic composition. It has been shown that the storage and sample preparation for extraction, the soil texture, and the soil water content, as well as the organic matter and carbonate content, strongly influence the final results (West et al., 2006; Wassenaar et al., 2008; Koeniger et al., 2011; Meißner et al., 2014; Hendry et al., 2015; Orlowski et al., 2016a; Newberry et al., 2017). Parallelly, the specifics of the extraction methods, e.g. the different pore spaces that may or may not be extracted via the different approaches (Orlowski et al., 2019; Kübert et al., 2020), and the modifications of the procedures themselves (Orlowski et., 2018) can affect the isotope results.
There are several classes of different extraction methods; some of them were compared in Zhu et al. (2014), Sprenger et al. (2015), and Orlowski et al. (2016b, 2018). In brief, there are methods using the following:
- a.
various chemical compounds or elements like toluene for azeotropic distillation (Revesz and Woods, 1990; Thorburn et al., 1993), dichloromethane for accelerated solvent extraction techniques (Zhu et al., 2014), and/or zinc or uranium for micro-distillation (Kendall and Coplen, 1985; Brumsack et al., 1992);
- b.
microwave water extraction (Munksgaard et al., 2014);
- c.
force in terms of mechanical squeezing (Wershaw et al., 1966; White et al., 1985; Böttcher et al., 1997) or centrifugation (Mubarak and Olsen, 1976; Batley and Giles, 1979; Barrow and Whelan, 1980; Peters and Yakir, 2008);
- d.
equilibration methods such as in situ equilibration (Garvelmann et al., 2012; Rothfuss et al., 2013, 2015; Volkmann and Weiler, 2014; Gaj et al., 2016), CO2 and H2 equilibration (Jusserand, 1980; Scrimgeour, 1995; Hsieh et al., 1998; McConville et al., 1999; Koehler et al., 2000; Kelln et al., 2001), and the direct liquid–vapour equilibrium laser spectroscopy (DVE-LS) method (Wassenaar et al., 2008; Hendry et al., 2015);
- e.
cryogenic vacuum extraction (CVE) (Dalton, 1988; West et al., 2006; Koeniger et al., 2011; Goebel and Lascano, 2012; Orlowski et al., 2013, 2016a; Gaj et al., 2017), the modified CVE–He-purging method (Ignatev et al., 2013), and the automatic cryogenic vacuum distillation (ACVD) system LI-2100 (Lica United Technology Limited Inc.).
In addition, many laboratories use various modifications of these methods (Walker et al., 1994; Munksgaard et al., 2014; Orlowski et al., 2018). A more detailed description of the above-stated methods is presented in Sprenger et al. (2015) and Ceperley et al. (2024).
At present, DVE-LS and CVE are the most commonly used methods for soil water extraction. Both methods provide very accurate results but only under specific conditions. For the DVE-LS method, the different equilibration times, low water content, and selection of bags play a crucial role (Hendry et al., 2015; Gralher et al., 2016). It has also been shown that soil samples with a high content of fine particles – and, thus, high soil tension – can cause isotope fractionation in closed systems (Gaj and McDonell, 2019). For the CVE method, the major challenge is the treatment of soils containing clay minerals. Such soils require an application of higher temperatures (up to 300 °C). However, this results in the release of water by oxidation of organics and dihydroxylation of hydroxide-containing minerals such as goethite (Gaj et al., 2017) and, in such a way, affects the experimental results. Moreover, the soil sample size acceptable for this method is rather low, usually between 10 to 20 g, allowing for the extraction of only grams of the soil water. Another disadvantage of the CVE method consists of the incomparable outputs among different laboratories due to the CVE setup modifications and different workflows (Orlowski et al., 2018). Laboratories' differences in terms of their setups are as follows: the extraction containers (form, size, volume, and material), the heating module and its working temperature (heating tapes or lamps, water baths or hot plates), the type of fittings and connections (glass, stainless steel), and the vacuum-producing units. In addition, different temperatures, pressures, extraction times, and sample sizes are applied by different laboratories. However, if a certain setup of all these parameters for the given situation is chosen, very accurate results can be achieved for certain soil types and water contents. Nevertheless, each of these two methods exhibits apparent inconveniences:
-
in the case of the DVE-LS method, significant time consumption (a requirement of the permanent presence of an operator);
-
in the case of the CVE method, an application of technically complicated methods (work with liquid nitrogen, low pressures, and high temperatures in an open laboratory apparatus).
In this study, we present a new extraction method – circulating-air soil water extraction (CASWE). It is a relatively simple, inexpensive method that can handle soil samples of different sizes, moisture contents, and textures. It is based on the simple principle of complete evaporation and condensation in a closed circuit and does not require an application of hazardous substances (acids, toluene, liquid nitrogen) and/or high temperatures and pressures. In the following we (1) introduce a new extraction principle, (2) present the results of soil extraction efficiency testing, and (3) compare the results with other state-of-the-art approaches. The advantage of the proposed method over the others is its accuracy, even with clay samples, known for causing inaccurate results for other extraction methods (Ceperley et al., 2024). The biggest advantages of this extraction method are as follows:
- a.
the high accuracy of the results,
- b.
the simple design and low cost of the apparatus setup,
- c.
the low operating costs,
- d.
the time reduction in operating the device, and
- e.
the ability to process large soil samples and thus obtain large and representative quantities of soil water.
2.1 Principle of extraction
The CASWE method is based on the principle of complete evaporation and subsequent condensation of soil water in a closed circuit using air as the circulating medium. The soil sample is heated inside the evaporation chamber to 105 °C, and the evaporated soil water is carried by air circulation to a cooling unit, where the water vapour condenses, and finally, the liquid water is collected. Dried cool air is then circulated back into the evaporation chamber. The process continues until no air moisture condensation is visible.
The extraction temperature was chosen based on the standard Czech methodology for soil drying (ISO 11 465, 1998), which is consistent with standard methodologies used in the UK (BSI 1377: 105 ± 5 °C) and the US (ASTM D2216: 110 ± 5 °C). Values exceeding 100 °C have to be chosen as pore water remains in the soil when temperatures below 100 °C are used (O'Kelly, 2004, 2005). The water vapour is then condensed using tap water at a temperature of 8 °C. The usage of tap water for cooling is motivated by the following reasons:
- a.
its availability;
- b.
the temperature of the cooling water is close to the ambient-air dew temperature (preventing the appearance of ambient-air condensation on the cooling loops and, hence, possible sample contamination);
- c.
the prevention of frost formation inside the apparatus, which otherwise increases the risk of blocking the inlet pipes, damaging the glass parts, and causing difficulty in extracted-sample handling (prior to the sample handling, frost on the cooler and collecting vessel walls has to be melted);
- d.
with respect to the vapour pressure at the extraction temperature (105 °C, 121 kPa), there is no apparent difference in terms of the extraction rates or residual soil moisture at the point of equilibrium with the cooling circuit operated at 8 °C (1 kPa) or −10 °C (0.3 kPa).
2.2 Description of the apparatus
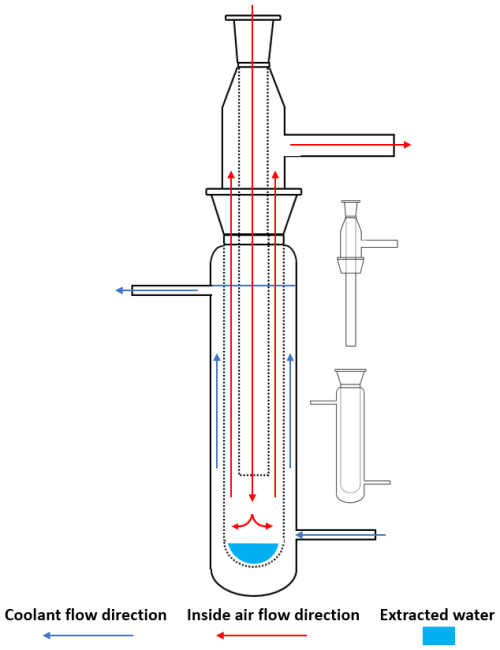
The newly designed apparatus (Fig. 1a) is composed of three main system units – heating, cooling, and air circulation (Fig. 2). The apparatus has four separate circuits for simultaneous water extraction from four different soil samples.
The heating system comprises a standard kitchen oven (model VT 332 CX; MORA MORAVIA, s.r.o., Czechia) housing four evaporation chambers made of stainless steel boxes equipped with airtight insulation. Each box has two openings, one for a dry-air inlet and the other for a moist-air outlet. The soil sample inside the box is placed on a stainless steel wire mesh bed providing good contact between the sample and the air, which enhances the water evaporation rate (Fig. 1b). The dry air is led to the evaporation chamber through a silicone rubber tube coiled inside the oven; its length (∼ 2 m) is sufficient to preheat the air to be close to the oven temperature (Fig. 1c). The hot and moist air from the evaporation chamber is led through the insulated silicone tube to the cooling system; the length of the outlet tubes is as short as possible to minimize the heat losses and to prevent undesired water condensation. To monitor the extraction process, a temperature sensor is installed inside each box close to the air outlet.
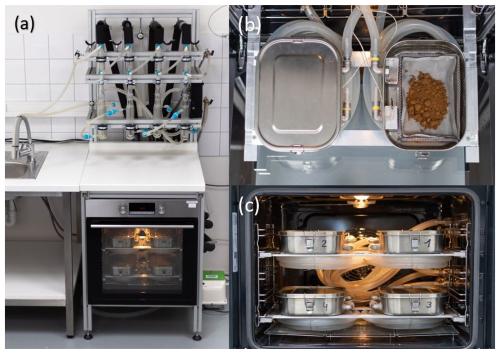
Figure 1(a) Photo of the proposed CASWE apparatus. (b) Details of the heating chamber with wire mesh bed and aluminium fabric bedding. (c) Internal arrangement of heating chambers and coiled supply hoses.
The cooling system consists of three glass components – a spiral cooler, a custom-made connecting part, and a jacketed collecting vessel (Fig. 3). Two separate cooling-water circuits are used for the spiral coolers and for the collecting vessels (Fig. 2).
The cooled and dried air from the cooling system is fed back to the evaporation chamber by means of the air circulation system, comprised of two regulated high-speed fans per circuit, ensuring the air flow rate of ∼ 10 L min−1. The temperature sensors and fan speed in each circuit are monitored by the control unit running on the Arduino platform. The apparatus is complemented by an air diaphragm pump that can be connected to any circuit to flush the circuit with fresh dry air to remove possible residual moisture in the apparatus prior to extraction, thus achieving more accurate results. The tests presented in this work were carried out without the use of this pump. However, for the extraction of soil water with significantly different isotopic compositions, the execution of an initial purge between extractions would be appropriate.
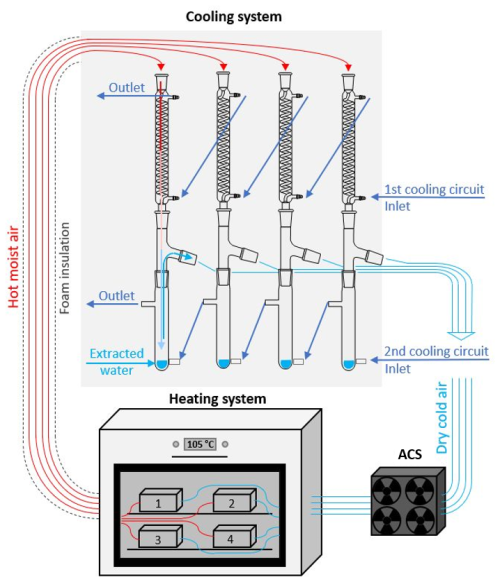
Figure 2A simplified diagram of the three main components of the CASWE apparatus (heating, cooling, and air circulation systems (ACSs)). The apparatus consists of four separate drying circuits and two cooling circuits.
2.3 Extraction procedure
Soil samples are inserted on the wire bed of the evaporation chambers. A target temperature of 105 °C is reached within approximately 15 min. This initiates the first intensive part of the drying process, during which both cooling circuits operate, with most of the water being extracted. The upper cooling circuit (Fig. 2) is disconnected once the spiral cooler starts to dry out. The extraction continues with the bottom cooling circuit only. During this time, residual moisture in the apparatus is collected in the cooled collection vessel.
The extraction is complete when there are no visible signs of condensation anywhere other than in the collection vessel. To check the completeness of the extraction, the recovery ratio was calculated for each extraction by comparing the weights of the added and extracted waters. For complete checking of the functionality of the apparatus, some soil samples were weighed after pre-oven-drying and after extraction. Depending on the sample type, water content, and size, the extraction time intervals ranged from 3 to 6 h per sample. Between each extraction, the circuit is disassembled to retrieve the extracted water from the collection vessel and to exchange soil samples. Thorough mixing of the sample before pouring from the collection vessel and catching of all of the droplets from the walls to ensure the homogeneity of the sample are needed. The collection vessel must then be dried to avoid contamination during further extraction.
2.4 Functional tests
In total, six functional tests were performed. All of the tests were aimed at recovering the same amount of water that was used with no changes in its isotopic composition. The first test served to provide a verification of the principle of extraction and to check the waterproofing and airtightness of the apparatus. The remaining five tests verified the accuracy of the extraction with soil samples via spike experiments. In these experiments, disturbed-soil samples were pre-oven-dried (105 °C for 24 h), spiked in the evaporation chamber with a specific amount of labelled water, mixed, and then left to equilibrate for 2 h. Five sets of spike experiments with different soil textures were performed as soil texture plays a crucial role during soil water extraction (Orlowski et al., 2016a). In each spike experiment, identical samples were rewetted repeatedly (with the exception of artificially prepared sandy clay, which is described below) to reveal any shift in the isotopic composition of the extracted water and, thus, to eliminate any possible influence of the residual water from the sample due to incomplete drying prior to extraction. This follows a procedure described in Gaj et al. (2017).
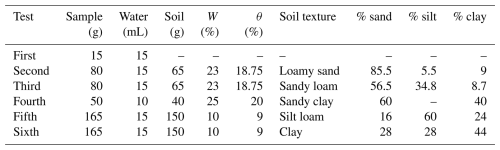
Six consequent tests (Table 1) were carried out in the following way:
-
First test. Water of known isotopic composition and quantity (15 mL) was poured into the heating chambers.
-
Second test. Disturbed-soil samples (65 g each) with a texture of loamy sand were spiked with 15 mL of water of known isotopic composition. The soil samples were reused and re-hydrated thrice.
-
Third test. The procedure was the same as in the second test but using sandy loam soil samples.
-
Fourth test. Samples weighted at 40 g were prepared in the laboratory by mixing sand (60 %) with clay (40 %) and spiking with 10 mL of known isotopic composition. A lower sample size and water amount were used to reduce the corresponding extraction time. In this case, a new sample was prepared for each extraction run due to concerns of possible sealing of the sample after extraction, which would make it difficult to re-hydrate.
-
Fifth and sixth tests. These tests were used to verify the functionality of the method with a lower water content (10 %). For the fifth test, disturbed-soil samples (150 g each) with a texture of silt loam were spiked with 15 mL of water of known isotopic composition. Since we did not observe any significant sealing in the previous test, the soil samples were reused and re-hydrated twice. The same procedure was used for the sixth test, only with a different soil texture (clay), with the samples being reused and re-hydrated thrice.
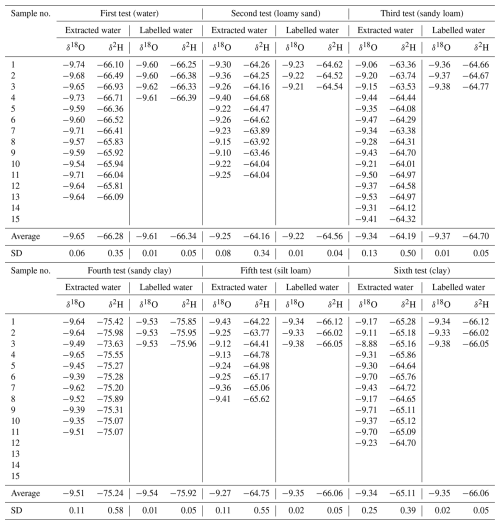
For each test, we employed labelled water that differed slightly in terms of the stable isotope composition (Table 2), analysed at the Institute of Hydrodynamics (Czech Academy of Sciences) with the L2140-i isotope analyser (Picarro Inc., US). The standard mode (precision of ± 0.03 ‰ and ± 0.15 ‰ for δ18O and δ2H, respectively) was used, with six injections per sample and with the first three injections being discarded. The isotope ratios are reported in per mil (‰) relative to Vienna Standard Mean Ocean Water (VSMOW) (δ2H or δ18O = () × 1000 ‰, where Rsample is the isotope ratio of the sample, and Rstandard is the known reference value (i.e. VSMOW, Craig, 1961). The target accuracy of the method is given by the limit of ± 0.2 ‰ for δ18O and ± 2 ‰ for δ2H, which is considered to be reasonable for hydrologic studies (Wassenaar et al., 2012; Orlowski et al., 2016b). The terms “shift” and “bias” were used for an evaluation of the results, where shift means a difference compared to the labelled water, and bias indicates the standard deviation of the data. Please note that these terms are often replaced by the terms accuracy (shift) and precision (bias) in some studies (Revesz and Woods, 1990; Koeniger et al., 2011; Ignatev et al., 2013; Zhu et al., 2014; Sprenger et al., 2015; Gaj et al., 2017).
3.1 Waterproof and airtightness test
To test the extraction method and the water- and air-tightness of the apparatus (first test), 15 mL of water of known isotopic composition was used. Extraction of this water amount took, on average, 5 h. The resulting recovery ratio after the extraction process averaged 99.7 % of the volume of the used labelled water. The remaining water fractions were given by the sum of the thin residual layer of moisture left on the walls inside the collection vessel during the transfer of the samples into the vials, the residual moisture inside the apparatus, and possible diffusion through the silicon tubing. The stable isotope composition of the labelled water used for this test was −9.61 ± 0.01 ‰ for δ18O and −66.34 ± 0.05 ‰ for δ2H (N=4) (Table 2, Fig. 4). The total average of the mean stable isotope composition of extracted water (N=13) was shifted by −0.04 ‰ (bias ± 0.06 ‰) and 0.06 ‰ (bias ± 0.35 ‰) for δ18O and δ2H, respectively.
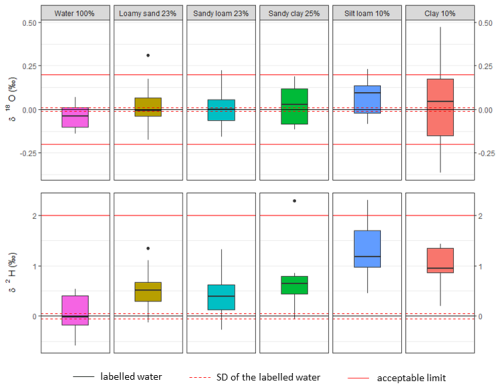
Figure 4Relative deviation of the isotopic ratio of extracted water compared to the labelled water and its standard deviation. For better clarity, all results are recalculated as if the used labelled water had a VSMOW composition. The acceptable limits are represented by the errors of ± 0.2 ‰ for δ18O and ± 2 ‰ for δ2H, which is considered to be reasonable for hydrologic studies (Wassenaar et al., 2012; Orlowski et al., 2016b).
3.2 Spike experiments
The other five tests – the spike experiments – verifying the functionality of the extraction took, on average, 3 h for the loamy sand, 4 h for the sandy loam, 5 h for the sandy clay and silt loam, and 6 h for the clay samples. The resulting recovery rate after the extraction process attained, on average, 99.3 % of the used labelled water volume (Table 2). The remaining water fractions were given, analogously to before, by the sum of the thin residual layer of moisture left on the walls inside the collection vessel during the transfer of the samples into the vials, the residual moisture inside the apparatus, and the possible diffusion through the silicon tubing. The sixth test represented the only exception (clay soil from the Halaba area, central Ethiopia), where the recovery rate often exceeded 100 %. Since a similar phenomenon was not observed with the other samples and because the apparatus was tested for possible leakage (which was not found), we hypothesize that this error is due to the extreme chemical composition of the selected samples (potential release of crystalline water from the soil itself) or insufficient pre-oven-drying (despite being applied for 72 h).
In the second test (loamy sand), the stable isotope composition of labelled water was −49.22 ± 0.01 ‰ for δ18O and −64.56 ± 0.04 ‰ for δ2H (N=3). The average obtained isotopic composition was depleted by 0.03 ± 0.08 ‰ in δ18O and enriched by 0.4 ± 0.34 ‰ in δ2H (N=11) (Table 2, Fig. 4). As in the first test, the δ18O values were slightly depleted but almost matched the labelled water. However, the δ2H values were relatively enriched (Fig. 4; Table A2 in the Appendix).
Table 2Summary of the individual test results.
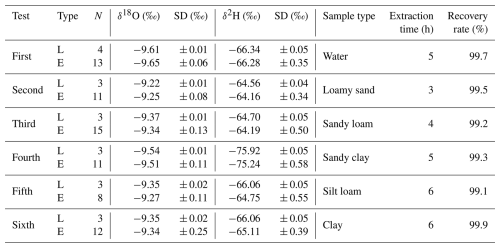
L and E indicate the labelled and extracted water used in the test, respectively. N stands for the number of samples. The isotope ratios (δ18O, δ2H) and their standard deviations (SD) are reported in per mil (‰) relative to the Vienna Standard Mean Ocean Water (VSMOW). The extraction times quoted are average times valid for the disturbed-soil samples and may vary with other samples depending on the sample size, texture, and water content. The recovery ratio was calculated as the weight of extracted water divided by the weight of the added labelled water, multiplied by 100.
In the third test (sandy loam), the stable isotope composition of the labelled water was −9.37 ± 0.01 ‰ for δ18O and −64.70 ± 0.05 ‰ for δ2H (N=3). The mean isotope composition of the extracted water was enriched for both isotopes but with no statistical significance for δ18O (Table A2). The average shift and bias attained 0.03 ± 0.13 ‰ for δ18O and 0.51 ± 0.5 ‰ for δ2H (N=15). Compared to the second test, the variance of the values increased.
In the fourth test (sandy clay), the stable isotope composition of the labelled water was −9.54 ± 0.01 ‰ for δ18O and −75.92 ± 0.05 ‰ for δ2H (N=3). The mean isotope composition of the extracted water was enriched for both isotopes but with no statistical significance for δ18O. The values of δ18O increased by 0.03 ± 0.11 ‰, and those of δ2H increased by 0.68 ± 0.58 ‰ (N=11).
In the fifth test (silt loam), the stable isotope composition of the labelled water attained −9.35 ± 0.02 ‰ for δ18O and −66.06 ± 0.05 ‰ for δ2H (N=3). The mean isotope composition of the extracted water was enriched for both isotopes but with no statistical significance for δ18O. The values of δ18O increased by 0.07 ± 0.11 ‰, and those of δ2H increased by 1.31 ± 0.55 ‰ (N=8).
In the sixth test (clay), the same labelled water was used as in the fifth test. The mean isotope composition of the extracted water was enriched for both isotopes but with no statistical significance for δ18O. The values were shifted by 0.01 ± 0.25 ‰ for δ18O and by 0.96 ± 0.39 ‰ for δ2H (N=12).
The Kolmogorov–Smirnov test at a 5 % significance level was performed for all sets of the results to determine the normality of the data. The measured data for all six tests exhibited a normal distribution. Furthermore, one sample t test was performed at a 5 % significance level to determine whether the extracted values were significantly different from the standard used in the given test. For the first set of the results (extraction test with water only), the average of the data is not statistically different from the standard used. In the remaining extraction tests, using soil, the mean is always statistically identical to the standard used only in the case of δ18O. In the case of δ2H values, the null hypothesis was always rejected. Furthermore, the data variance of δ2H increases with a higher number of fine particles in the soil (silt, clay). The statistical test results are summarized in Table A2.
Since the normality test, which is a prerequisite for the t test, may not be valid for such small data sets, we also performed the bootstrap analysis, which does not require this assumption. This analysis calculates the 95 % confidence interval in which the true value is located (Fig. A1). The results of this analysis were consistent with the results of the t test.
4.1 Residual moisture in the apparatus
The apparatus is designed to handle an entire standard soil core (100 cm3). The sample size is limited only by the volume of the heating chamber (roughly 400 cm3 of usable space) and the size of the collection vessel (∼ 25 mL). An advantage of extracting a bigger soil sample over extracting the smaller ones (e.g. < 10 g) is a much better representation of the sample properties. The larger amount of obtained extracted water with the proposed extraction apparatus lowers the potential inaccuracy which accompanies lower sampling amounts in other extraction methods. Additionally, it offers the advantage of being able to run the same extracted water sample using both the isotope ratio mass spectrometry (IRMS) and the isotope ratio infrared spectroscopy (IRIS) machines. However, not all water ends up in the collection vessel. Based on the estimated gas volume of 4 L, the ideal gas law, and the equilibrium conditions at 8 °C, the amount of water left in the circuit is approximately 50 mg. Furthermore, humidity gains and losses can occur during the extraction procedure because of the silicon hoses' permeability. The estimates of humidity losses for the extraction time not exceeding 24 h are less than 0.5 % of the total sample mass, regardless of the extracted water amount. The estimates are based on the water–silicone solubility and permeability (Barrie and Machin, 1969), supposing 50 % relative humidity in the room outside the extractor and 8 °C cooling water. Under these conditions, the absolute air humidity inside the extractor is higher (during the proceeding extraction) or equal to the ambient-air humidity, allowing for minor sample losses (< 0.5 %) via vapour permeation when the extraction proceeds and no losses once the sample is almost or completely dry. The hoses can also absorb water vapour from the air. The water absorbed in the silicone hoses is released back into the circuit when heated (by calculation, estimated to be approximately 50 µg). Although silicone hoses may not seem ideal for this purpose, the choice of construction materials was a compromise between handling and operating the extractor and material resistance or neutrality with respect to the extracted water. Despite the potential sample gains or losses, these amounts are still marginal compared to the amount of extracted water and so do not exhibit a major effect on the results (as demonstrated).
Most of these error sources can be suppressed by using larger sample sizes. For even more accurate results, it might help to choose a different construction material (PTFE, stainless steel), to seal the apparatus entirely during idle time, to pre-dry the empty apparatus, or to purge the apparatus with dry air or nitrogen (as inert gas). However, the extraction procedure would be more complicated, and the nuances that this would resolve are negligible in comparison to other factors (e.g. the amount of clay in the sample and the accuracy of measurements of the stable isotope composition itself) which will affect the final composition more significantly.
Thorough mixing of the sample before pouring from the collection vessel and catching all droplets from the walls to ensure the homogeneity of the sample is necessary. Because of that, the water adheres to the walls of the collecting vessel, whereby the residual amount always remains there while the sample is poured into the vials. This adhered water contributes significantly to the incomplete recovery rate and often covers the majority of this error. Since the sample was mixed (homogenized) during the collection of all residual droplets on the walls of the collection vessel, we assume that the residual film in the glass will not affect the isotopic composition of the sample but, rather, will only affect the recovery ratio.
With respect to the Rayleigh distillation principle (Dansgaard, 1964; Araguás-Araguás et al., 1995), the observed shift of extracted soil water towards enriched values of the heavier isotopes also points to imperfect collection of extracted water. The slight enrichment indicates incomplete water condensation and the presence of lighter isotopes (as quantified above) inside the apparatus, as also evidenced by the high but incomplete recovery rate. Complete evaporation of the soil water is confirmed by comparison of the soil sample weights (the weight after extraction for selected samples was equal to or slightly lower than the sample weight after pre-oven-drying).
As discussed earlier, two factors, outlined below, can notably influence the composition of the collected water and, thus, the reliability of the proposed method: insufficient tightness of the whole circuit (joints, etc.) and permeability of the pipes made of silicon. The absence of the former factor is checked by the recovery rate being close to 100 %. The latter factor – possible sample contamination with ambient moisture comprising substantially lighter isotopic compositions (∼ −13 ‰ and −125 ‰ for δ18O and δ2H, respectively) – is almost completely suppressed as the experimental results exhibit only negligible changes in the labelled water isotopic composition. Moreover, the observed shift in the water composition (enrichment by heavier isotopes) indicates marginal sample fractionation instead of its contamination by ambient moisture.
4.2 Extraction time
For many methods, extraction time often plays a significant role in the resulting isotopic composition of the sample (Revesz and Woods, 1990; West et al., 2006; Zhu et al., 2014; Hendry et al., 2015; Orlowski et al., 2018; Orlowski and Breuer, 2020). In this case, no significant differences were observed between ending the extraction at the time when the circuit is visibly dry and prolonging the extraction by an hour or more because the same dry, cold air is still flowing when the extraction is completed. Once the extraction is complete, the apparatus reaches an equilibrium state in which the amount and composition of the water sample are fixed. The proposed method is one of the slower ones compared to other extraction methods. The extraction time using the CVE method varies from 15 min (Orlowski et al., 2018) to 6 h (Mora and Jahren, 2003). However, it should be added that, for the CVE method, sample sizes of 10–20 g are used, and only a few millilitres of water is extracted (Table 3), whereas, in the presented method, extraction of the sample size attained up to 150 g and extracted liquid-water amounts of up to 15 mL. The extraction time is therefore longer and varies between 3 to 6 h depending on the soil texture (the larger porosity of the sample reduces the extraction time significantly), water content, and sample size. The presence of pores in the soil and, thus, the larger surface area for evaporation also constitute the reason why the extraction time of some soil samples was shorter than the extraction of water alone (first test). The soils are dried on a manufactured bed to allow air to reach the soil sample from all sides. Contrarily, the water sample was placed in a small stainless steel bowl, enabling air–water interaction only on the surface (upper side). By making this surface larger for the soil, the extraction is faster. Also, the soil itself exhibits a higher thermal conductivity than air.
In the case of low soil moisture, a larger soil sample should be used (to extract at least 7–10 mL of water), resulting in a longer extraction time. The extraction times quoted above are average times valid for the samples used in this study and may vary with other samples (especially undisturbed samples or samples with different water contents).
Table 3Comparison of the reported results of selected soil water extraction methods in different studies.
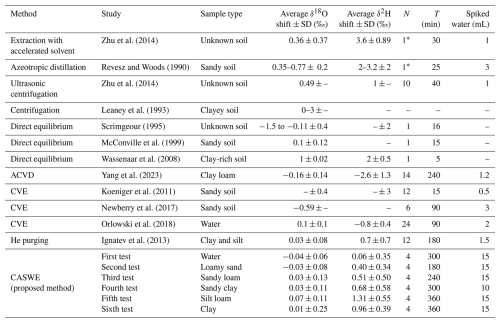
The values represent the average shift from the labelled water used ± the standard deviation. ACVD stands for automatic cryogenic vacuum distillation, CVE stands for cryogenic vacuum extraction, and CASWE stands for circulating-air soil water extraction method. CVE results from the study by Orlowski et al. (2018) show only the best results achieved in the comparison of CVEs conducted in that study. Average δ18O and δ2H shifts represent the deviation from the mean of the used labelled waters. SD stands for standard deviation (bias). T is the extraction time for N samples that can be processed simultaneously. The number of samples marked with * may vary depending on the size of the apparatus. The last column gives the amount of labelled water used.
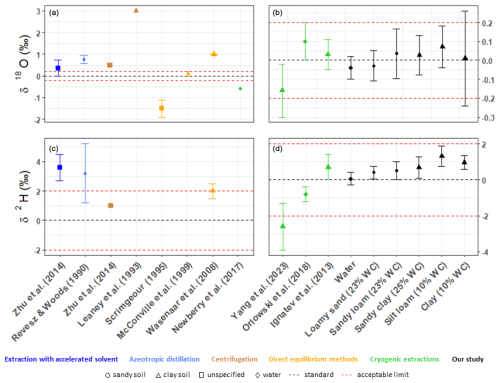
Figure 5A graphical comparison of the presented results with other methods (a, b for δ18O; c, d for δ2H). Different markings indicate different sample types. The acceptable limits are represented by the errors of ± 0.2 ‰ for δ18O and ± 2 ‰ for δ2H, which are considered to be reasonable for hydrologic studies (Wassenaar et al., 2012; Orlowski et al., 2016b). The right side of the oxygen graph (b) with more accurate methods has a zoomed-in y axis.
In large-scale studies, higher sample throughput is an important factor. For these purposes, apparatuses with higher throughput that can handle 30 or more samples in an 8 h working day are used (Goebel and Lascano, 2012; Orlowski et al., 2013; Yang et al, 2023). The proposed apparatus currently has only four circuits; hence, four soil samples can be processed simultaneously. Depending on the soil type and water content, a maximum of two runs per day can be processed. Reduction of the sample size could increase the throughput, resulting in a reduction of the extraction time, but this could be projected to lead to higher inaccuracy in the results.
4.3 Comparison of soil water extraction approaches
In order to compare the proposed method of soil water extraction with other approaches, we gathered the results presented in other references. The results proved (Table 3 and Fig. 5) that the presented method is able to fit safely within an acceptable range of accuracy (± 0.2 ‰ for δ18O and ± 2 for δ2H ‰; Wassenaar et al., 2012), which is, for other methods, rather problematic even if different soil types are used. For example, with a clay-rich soil sample, the DVE-LS method (Wassenaar et al., 2008) achieves low standard deviations (± 0.02 ‰ and ± 0.5 ‰ for δ18O and δ2H, respectively), but the shift in the data is at (+2 ‰ for δ2H) or beyond (+1 ‰ for δ18O) the limit of acceptability. McConville et al. (1999) obtained very accurate results with the direct-equilibrium method (0.1 ± 0.12 ‰ for δ18O), but only a sandy soil was studied. A comparison with the most commonly used method, CVE, is difficult due to the huge dispersion of values presented by different laboratories (Orlowski et al., 2016b, 2018). In this study, we used the reported values of Yang et al. (2023), Newberry et al. (2017), and Koeniger et al. (2011) as a reference. The reported shifts in the data were between −0.16 ‰ to −0.59 ‰ and −2.6 ‰ to 2 ‰ for δ18O and δ2H, respectively, and the deviation was in the range of ± 0.14 ‰ to 0.4 ‰ and ± 1.3 ‰ to 3 ‰ for δ18O and δ2H, respectively, where the most problematic samples exhibited a high content of clay particles. Based on the tests we have carried out so far, it seems that, in some cases, the obtained shifts are up to 1 order of magnitude lower than the shifts in the above studies. The reported values are depleted in both isotopes, which contradicts the values reported in this study (where, in particular, the δ2H values are rather enriched). Orlowski et al. (2016b) showed that, in the case of extraction from sandy samples, the water extracted by means of the CVE method is almost identical to the applied label water. However, as the proportion of clay particles in the sample increases, the accuracy decreases significantly, and the difference compared to the labelled water for clay samples is more than 1.5 ‰ and 12 ‰ for δ18O and δ2H, respectively. In this study, with an increasing amount of clay in the sample, only a gradual shift in isotopic composition is visible. For both isotopes, there is a higher enrichment of heavy isotopes in the sample, and the dispersion of the values increases. Only the δ2H is statistically different from the labelled water used (Table A2, Fig. A1).
Many laboratories have considerable problems with the extraction of water itself (Orlowski et al., 2018). The best-reported results of extracted water in the interlaboratory study by Orlowski et al. (2018) were 0.1 ± 0.1 ‰ for δ18O and −0.8 ± 0.4 ‰ for δ2H, which, again, differed by almost 1 order of magnitude from the results presented in this study. Only 2 out of 16 laboratories in the CVE interlaboratory comparison study presented comparable results. This indicates that the problem with accuracy is not caused by the method itself (CVE can give very accurate results) but is rather connected with the possibility of how to arrange the settings of the apparatus. Minor differences may occur due to the measurement of the isotopic composition itself depending on the instrument and method used (Penna et al., 2010, 2012).
The method providing comparable results with this study is a modification of the CVE method presented by Ignatev et al. (2013), which uses He as carrier gas instead of water vapour diffusion only. In both cases, mass transfer coupled with gas flow (air in the presented study and He in Ignatev et al.'s case) was shown to be more efficient compared to diffusive mass transfer (Ishimaru et al., 1992), and, hence, more accurate results can be achieved. The reported values by Ignatev et al. (2013) are 0.03 ± 0.08 ‰ and 0.7 ± 0.7 ‰ for δ18O and δ2H, respectively. In comparison with the proposed method, there is a higher shift in δ18O values but a lower shift in δ2H values in the He-purging method. However, it should be noted that these differences of hundredths (δ18O) to units of tenths (δ2H) are mostly within the measurement inaccuracy of an isotope analyser.
In our opinion, another step possibly affecting the CVE results (that is not present in the proposed procedure) is the actual vacuum formation in the CVE apparatus. Although the soil sample is inserted into the apparatus frozen in the prevailing majority of cases, there is no guarantee that evaporation or sublimation does not occur at very low pressures.
4.4 Limitations of the proposed method and future development
The main advantage of the CASWE method consists in its ability to extract water from relatively large (hundreds of grams) soil samples, which reduces the effect of soil heterogeneity and sample handling on the measured isotopic composition. On the other hand, such a sample size brings about some less favourable effects. In the field, the commonly used narrow soil probes may be insufficient to collect the required amount of soil, which may necessitate the excavation of a larger pit to obtain a sample. This makes the process more complex and time-consuming, and extra care must be taken to prevent isotopic fractionation of the collected samples. Moreover, the larger amount of soil causes a longer extraction time. In this manner, the complications with handling large soil samples restrict the CASWE method utilization.
For broader use, it would be necessary to change the apparatus design in order to simultaneously enable parallel treatment of more samples and to reduce the extraction time. The latter could be achieved by increasing the air flow circulation rate and reducing the premature condensation inside the pipeline by, for example, improved insulation or additional heating of the tubes. The apparatus could be adapted for medium-scale studies by choosing a different source of heating and by inserting additional evaporation chambers.
A new method for soil water extraction – circulating-air soil water extraction (CASWE) – is presented, and a new apparatus is developed for its purposes. The method works on the principle of complete evaporation and condensation in a closed circuit. The soil water was successfully extracted from dried and rehydrated soil samples of different textures (loamy sand, sandy loam, sandy clay, silt loam, and clay). Depending on the soil texture, the average shift from the labelled water used ranged between −0.04 ‰ and 0.07 ‰ for δ18O and between 0.4 ‰ and 1.3 ‰ for δ2H, with the bias ranging from ± 0.08 ‰ to 0.25 ‰ and ± 0.34 ‰ to 0.58 ‰ for δ18O and δ2H, respectively. The differences between the extracted and used labelled water were often within the measurement error of the used isotope analyser. From the tests we have executed so far, we obtained results with lower shift than what is reported using other soil water extraction and/or equilibration methods, such as the CVE and DVE-LS methods, and with up to an order of magnitude lower shift compared to other methods (extraction with accelerated solvent, centrifugation, azeotropic distillation). This is achieved through the ability to process large soil samples, thereby reducing the effect of soil heterogeneity on the isotopic composition of extracted water and suppressing the inaccuracies accompanying the extraction process. However, the developed apparatus currently has a low throughput, with a maximum of eight samples per day, due to, besides its small capacity, the long extraction times. As a result, its use for fast processing of large sample quantities is limited. It is designed specifically for small-scale high-precision studies where unambiguous determination of the water origin is required. Also, it can be applied as a supplementary method for studies requiring high throughput, serving as a reference for the calibration of less accurate extraction methods. We believe that further development, leading to an increased throughput, could also enable the application of this method in medium-scale studies and could contribute to a deeper understanding of processes in the vadose zone.
This Appendix contains two additional tables and one figure. Table A1 shows all of the measured data from all of the functional tests. Table A2 presents the statistical results (test of variance, Kolmogorov–Smirnov test, and t test). Figure A1 depicts the results of the bootstrap analysis.
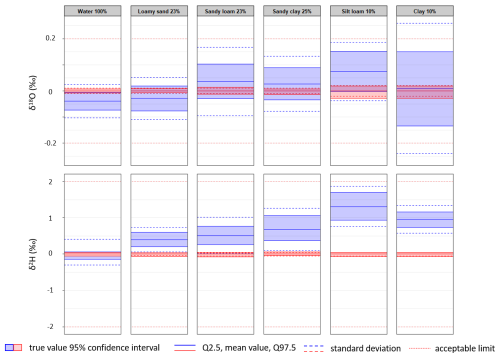
Figure A1The results of the bootstrap analysis. The blue colour represents the extracted values, and the red colour represents the standards used in these tests.
Table A2Statistical test results.
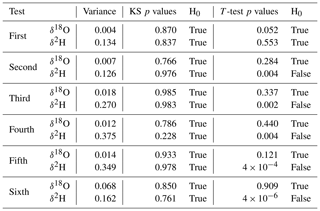
KS H0: the data set has a normal distribution. T-test H0: the sample mean is equal to the reference value. True denotes an acceptance of the null hypothesis, and false denotes a rejection of it. The values were rounded to three valid decimal figures, respecting the uncertainty of the experimental errors.
The code is available from the corresponding author upon request.
The data from this study are fully available in Table A1.
Concept: JH, OG. Methodology: JK, JH, OG. Software: JH, OG. Investigation: JK. Validation: JK, KF, VS, MS, LV. Visualization: JK. Writing (original draft preparation): JK, KF. Writing (review and editing): JK, JH, OG, KF, VS, MS, NO, LV. Supervision: LV.
At least one of the (co-)authors is a member of the editorial board of Hydrology and Earth System Sciences. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors warmly thank Petr Filip at the Institute of Hydrodynamics of the Czech Academy of Sciences for his help in the final revision of the paper. We are also grateful to three anonymous reviewers for their useful comments on an earlier version of the paper.
This work was supported by the Czech Academy of Sciences (grant no. RVO 67985874), the research programme Strategy AV21 Water for Life, the Czech Science Foundation (grant no. GA CR 22-12837S); the Faculty of Science of Charles University in Prague (grant no. SVV 244-2606941), and the German Federal Environmental Foundation (DBU).
This paper was edited by Genevieve Ali and reviewed by three anonymous referees.
Araguás-Araguás, L., Rozanski, K., Gonfiantini, R., and Louvat, D.: Isotope effects accompanying vacuum extraction of soil water for stable isotope analyses, J. Hydrol., 168, 159–171, https://doi.org/10.1016/0022-1694(94)02636-P, 1995.
Barrow, N. J. and Whelan, B. R.: A study of a method for displacing soil solution by centrifuging with an immiscible liquid, J. Environ. Qual., 9, 315–319, https://doi.org/10.2134/jeq1980.00472425000900020031x, 1980.
Batley, G. and Giles, M.: Solvent displacement of sediment interstitial waters before trace metal analysis, Water Res., 13, 879–886, https://doi.org/10.1016/0043-1354(79)90223-9, 1979.
Barrie, J. A. and Machin. D.: The sorption and diffusion of water in silicone rubbers: Part I. Unfilled rubbers, J. Macromol. Sci. B, 3, 645–672, https://doi.org/10.1080/00222346908217112, 1969.
Böttcher, G., Brumsack, H.-J., Heinrichs, H., and Pohlmann, M.: A new high-pressure squeezing technique for pore fluid extraction from terrestrial soils, Water Air Soil Pollut., 94, 289–296, https://doi.org/10.1007/BF02406064, 1997.
Brumsack, H., Zuleger, E., Gohn, E., and Murray, R.: Stable and radiogenic isotopes in pore waters from leg 127, Japan Sea, in: Proceedings of the Ocean Drilling Program, edite by: Pisciotto, K., Ingle, J., von Breymann, M., and Barron, J., Sci. Res., 127/128, 635–650, https://doi.org/10.2973/odp.proc.sr.127128-1.165.1992, 1992.
Ceperley, N., Gimeno, T. E., Jacobs, S. R., Beyer, M., Dubbert, M., Fischer, B., Geris, J., Holko, L., Kübert, A., Le Gall, S., Lehmann, M. M., Llorens, P., Millar, C., Penna, D., Prieto, I., Radolinski, J., Scandellari, F., Stockinger, M., Stumpp, C., Tetzlaff, D., van Meerveld, I., Werner, C., Yildiz, O., Zuecco, G., Barbeta, A., Orlowski, N., and Rothfuss, Y.: Toward a common methodological framework for the sampling, extraction, and isotopic analysis of water in the Critical Zone to study vegetation water use, WIREs Water, 11, e1727, https://doi.org/10.1002/wat2.1727, 2024.
Craig, H.: Standard for reporting concentrations of deuterium and oxygen-18 in natural waters, Science, 133, 1833–1834, https://doi.org/10.1126/science.133.3467.1833, 1961.
Dansgaard, W.: Stable isotopes in precipitation, Tellus, 16, 436–468, https://doi.org/10.1111/j.2153-3490.1964.tb00181.x, 1964.
Dalton, F. N.: Plant root water extraction studies using stable isotopes, Plant Soil, 111, 217–221, https://doi.org/10.1007/BF02139942, 1988.
Gaj, M. and McDonell, J. J.: Possible soil tension controls on the isotopic equilibrium fractionation factor for evaporation from soil, Hydrol. Process., 33, 1629–1634, https://doi.org/10.1002/hyp.13418, 2019.
Gaj, M., Beyer, M., Koeniger, P., Wanke, H., Hamutoko, J., and Himmelsbach, T.: In situ unsaturated zone water stable isotope (2H and 18O) measurements in semi-arid environments: a soil water balance, Hydrol. Earth Syst. Sci., 20, 715–731, https://doi.org/10.5194/hess-20-715-2016, 2016.
Gaj, M., Kaufhold, S., and McDonnell, J. J.: Potential limitation of cryogenic vacuum extractions and spiked experiments, Rapid Commun. Mass Sp., 31, 821–823, https://doi.org/10.1002/rcm.7850, 2017.
Garvelmann, J., Külls, C., and Weiler, M.: A porewater-based stable isotope approach for the investigation of subsurface hydrological processes, Hydrol. Earth Syst. Sci., 16, 631–640, https://doi.org/10.5194/hess-16-631-2012, 2012.
Goebel, T. S. and Lascano, R. J.: System for high throughput water extraction from soil material for stable isotope analysis of water, Journal of Analytical Sciences, Method. Instrum., 2, 203–207, https://doi.org/10.4236/jasmi.2012.24031, 2012.
Gralher, B., Herbstritt, B., Weiler, M., Wassenaar, L. I., and Stumpp, C.: Correcting Laser-Based Stable Isotope Reading Biased by Carrier Gas Changes, Environ. Sci. Technol., 50, 7047–7081, https://doi.org/10.1021/acs.est.6b01124, 2016.
Hendry, M. J., Schmeling, E., Wassenaar, L. I., Barbour, S. L., and Pratt, D.: Determining the stable isotope composition of pore water from saturated and unsaturated zone core: improvements to the direct vapour equilibration laser spectrometry method, Hydrol. Earth Syst. Sci., 19, 4427–4440, https://doi.org/10.5194/hess-19-4427-2015, 2015.
Hsieh, J. C. C., Savin, S. M., Kelly, E. F., and Chadwick, O. A.: Measurement of soil-water δ18O values by direct equilibration with CO2, Geoderma, 82, 255–268, https://doi.org/10.1016/S0016-7061(97)00104-3, 1998.
Ignatev, A., Velivetckaia, T., Sugimoto, A., and Ueta, A.: A soil water distillation technique using He-purging for stable isotope analysis, J. Hydrol., 498, 265–273, https://doi.org/10.1016/j.jhydrol.2013.06.032, 2013.
Ishimaru, H., Itoh, K., Ishigaki, T., and Furutate, M.: Fast pump-down aluminum ultrahigh vacuum system, J. Vacuum Sci. Technol., 10, 547–552, https://doi.org/10.1116/1.578186, 1992.
Jusserand, C.: Pore water extraction from sediment and soil: Oxygen-18 abundances comparisons between different techniques. First results, Catena, 7, 87–96, https://doi.org/10.1016/S0341-8162(80)80006-3, 1980.
Kelln, C. J., Wassenaar, L. I., and Hendry, M. J.: Stable Isotopes (δ18O, δ2H) of Pore Waters in Clay-Rich Aquitards: A Comparison and Evaluation of Measurement Techniques, Ground Water Monit. R., 21, 108–116, https://doi.org/10.1111/j.1745-6592.2001.tb00306.x, 2001.
Kendall, C. and Coplen, T. B.: Multi-sample conversion of water to hydrogen by zinc for stable isotope determination, Anal. Chem., 57, 1437–1440, https://doi.org/10.1021/ac00284a058, 1985.
Koehler, G., Wassenaar, L. I., and Hendry, M. J.: An Automated Technique for Measuring δD and δ18O Values of Porewater by Direct CO2 and H2 Equilibration, Anal. Chem., 72, 5659–5664, https://doi.org/10.1021/ac000498n, 2000.
Koeniger, P., Marshall, J. D., Link, T., and Mulch, A.: An inexpensive, fast, and reliable method for vacuum extraction of soil and plant water for stable isotope analyses by mass spectrometry, Rapid Commun. Mass Sp., 25, 3041–3048, https://doi.org/10.1002/rcm.5198, 2011.
Kübert, A., Paulus, S., Dahlmann, A., Werner, C., Rothfuss, Y., Orlowski, N., and Dubbert, M.: Water stable isotopes in ecohydrological field research: comparison between in situ and destructive monitoring methods to determine soil water isotopic signatures, Plant Sci., 11, 387, https://doi.org/10.3389/fpls.2020.00387, 2020.
Leaney, F. W., Smettem, K. R. J., and Chittleborough, D. J.: Estimating the contribution of preferential flow to subsurface runoff from a hillslope using deuterium and chloride, J. Hydrol., 147, 83–103, https://doi.org/10.1016/0022-1694(93)90076-L, 1993.
McConville, C., Kalin, R. M., and Flood, D.: Direct equilibration of soil water for δ18O analysis and its application to tracer studies, Rapid Commun. Mass Sp., 13, 1339–1345, https://doi.org/10.1002/(SICI)1097-0231(19990715)13:13<1339::AID-RCM559>3.0.CO;2-N, 1999.
Meißner, M., Köhler, M., Schwendenmann, L., Hölscher, D., and Dyckmans, J.: Soil water uptake by trees using water stable isotopes (δ2H and δ18O) – a method test regarding soil moisture, texture and carbonate, Plant Soil, 376, 327–335, https://doi.org/10.1007/s11104-013-1970-z, 2014.
Mora, G. and Jahren, A. H.: Isotopic evidence for the role of plant development on transpiration in deciduous forests of southern United States, Global Biogeochem. Cy., 17, 13-1–13-7, https://doi.org/10.1029/2002GB001981, 2003.
Mubarak, A. and Olsen, R.: Immiscible displacement of soil solution by centrifugation, Soil Sci. Soc. Am. J., 40, 329–331, 1976.
Munksgaard, N. C., Cheesman, A. W., Wurster, C. M., Cernusak, L. A., and Bird, M. I.: Microwave extraction–isotope ratio infrared spectroscopy (ME-IRIS): a novel technique for rapid extraction and in-line analysis of δ18O and δ2H values of water in plants, soils and insects, Rapid Commun. Mass Sp., 28, 2151–2161, https://doi.org/10.1002/rcm.7005, 2014.
Newberry, S. L., Prechsl, U. E., Pace, M., and Kahmen, A.: Tightly bound soil water introduces isotopic memory effects on mobile and extractable soil water pools, Isot. Environ. Healt. S., 52, 368–381, https://doi.org/10.1080/10256016.2017.1302446, 2017.
O'Kelly, B. C.: Accurate Determination of Moisture Content of Organic Soils Using the Oven Drying Method, Dry. Technol., 22, 1767–1776, https://doi.org/10.1081/DRT-200025642, 2004.
O'Kelly, B. C.: Oven-Drying Characteristics of Soil of Different Origins, Dry. Technol., 23, 1141–1149, https://doi.org/10.1081/DRT-200059149, 2005.
Orlowski, N. and Breuer, L.: Sampling soil water along pF curve for δ2H and δ18O analysis, Hydrol. Process., 34, 4959–4972, https://doi.org/10.1002/hyp.13916, 2020.
Orlowski, N., Frede, H.-G., Brüggemann, N., and Breuer, L.: Validation and application of a cryogenic vacuum extraction system for soil and plant water extraction for isotope analysis, J. Sensors Sensor Syst., 2, 179–193, https://doi.org/10.5194/jsss-2-179-2013, 2013.
Orlowski, N., Breuer, L., and McDonell, J. J.: Critical issues with cryogenic extraction of soil water for stable isotope analysis, Ecohydrology, 9, 3–10, https://doi.org/10.1002/eco.1722, 2016a.
Orlowski, N., Pratt, D. L., and McDonell, J. J.: Intercomparison of soil pore water extraction methods for stable isotope analysis, Hydrol. Process., 30, 3433–3449, https://doi.org/10.1002/hyp.10870, 2016b.
Orlowski, N., Breuer, L., Angeli, N., Boeckx, P., Brumbt, C., Cook, C. S., Dubbert, M., Dyckmans, J., Gallagher, B., Gralher, B., Herbstritt, B., Hervé-Fernández, P., Hissler, C., Koeniger, P., Legout, A., Macdonald, C. J., Oyarzún, C., Redelstein, R., Seidler, C., Siegwolf, R., Stumpp, C., Thomsen, S., Weiler, M., Werner, C., and McDonnell, J. J.: Inter-laboratory comparison of cryogenic water extraction systems for stable isotope analysis of soil water, Hydrol. Earth Syst. Sci., 22, 3619–3637, https://doi.org/10.5194/hess-22-3619-2018, 2018.
Orlowski, N., Pratt, D. L., and McDonnell, J. J.: Intercomparison of soil pore water extraction methods for stable isotope analysis and interpretation of hillslope runoff sources, Hydrol. Process., 33, 2939–2954, https://doi.org/10.1002/hyp.13539, 2019.
Penna, D., Stenni, B., Šanda, M., Wrede, S., Bogaard, T. A., Gobbi, A., Borga, M., Fischer, B. M. C., Bonazza, M., and Chárová, Z.: On the reproducibility and repeatability of laser absorption spectroscopy measurements for δ2H and δ18O isotopic analysis, Hydrol. Earth Syst. Sci., 14, 1551–1566, https://doi.org/10.5194/hess-14-1551-2010, 2010.
Penna, D., Stenni, B., Šanda, M., Wrede, S., Bogaard, T. A., Michelini, M., Fischer, B. M. C., Gobbi, A., Mantese, N., Zuecco, G., Borga, M., Bonazza, M., Sobotková, M., Čejková, B., and Wassenaar, L. I.: Technical Note: Evaluation of between-sample memory effects in the analysis of δ2H and δ18O of water samples measured by laser spectroscopes, Hydrol. Earth Syst. Sci., 16, 3925–3933, https://doi.org/10.5194/hess-16-3925-2012, 2012.
Peters, L. I. and Yakir, D.: A direct and rapid leaf water extraction method for isotopic analysis, Rapid Commun. Mass Sp., 22(18), 2929–2936, https://doi.org/10.1002/rcm.3692, 2008.
Revesz, K. and Woods, P. H.: A method to extract soil water for stable isotope analysis, J. Hydrol., 115, 397–406, https://doi.org/10.1016/0022-1694(90)90217-L, 1990.
Rothfuss, Y., Vereecken, H., and Brüggemann, N.: Monitoring water stable isotopic composition in soils using gas-permeable tubing and infrared laser absorption spectroscopy, Water Resour. Res., 49, 1–9, https://doi.org/10.1002/wrcr.20311, 2013.
Rothfuss, Y., Merz, S., Vanderborght, J., Hermes, N., Weuthen, A., Pohlmeier, A., Vereecken, H., and Brüggemann, N.: Long-term and high-frequency non-destructive monitoring of water stable isotope profiles in an evaporating soil column, Hydrol. Earth Syst. Sci., 19, 4067–4080, https://doi.org/10.5194/hess-19-4067-2015, 2015.
Scrimgeour, C. M.: Measurement of plant and soil water isotope composition by direct equilibration methods, J. Hydrol., 172, 261–274, https://doi.org/10.1016/0022-1694(95)02716-3, 1995.
Sprenger, M., Herbstritt, B., and Weiler, M.: Established methods and new opportunities for pore water stable isotope analysis, Hydrol. Process., 29, 5174–5192, https://doi.org/10.1002/hyp.10643, 2015.
Stumpp, C., Brüggemann, N., and Wingate, L.: Stable Isotope Approaches in Vadose Zone Research, Vadose Zone J., 17, 1–7, https://doi.org/10.2136/vzj2018.05.0096, 2018.
Thorburn, P. J., Walker, G. R., and Brunel, J.-P.: Extraction of water from Eucalyptus trees for analysis of deuterium and oxygen-18: laboratory and field techniques, Plant Cell Environ., 16, 269–277, https://doi.org/10.1111/j.1365-3040.1993.tb00869.x, 1993.
Volkmann, T. H. M. and Weiler, M.: Continual in situ monitoring of pore water stable isotopes in the subsurface, Hydrol. Earth Syst. Sci., 18, 1819–1833, https://doi.org/10.5194/hess-18-1819-2014, 2014.
Walker, G. R., Woods, P. H., and Allison, G. B.: Interlaboratory comparison of methods to determine the stable isotope composition of soil water, Chem. Geol., 111, 297–306, https://doi.org/10.1016/0009-2541(94)90096-5, 1994.
Wassenaar, L. I., Hendry, M. J., Chostner, V. L., and Lis, G. P.: High Resolution Pore Water δ2H and δ18O Measurements by H2O(liquid)-H2O(vapor) Equilibration Laser Spectroscopy, Environ. Sci. Technol., 42, 9262–9267, https://doi.org/10.1021/es802065s, 2008.
Wassenaar, L. I., Ahmad, M., Aggarwal, P., van Duren, M., Pöltenstein, L., Araguas, L., and Kurttas, T.: Worldwide proficiency test for routine analysis of δ2H and δ18O in water by isotope-ratio mass spectrometry and laser absorption spectroscopy, Rapid Commun. Mass Sp., 26, 1641–1648, https://doi.org/10.1002/rcm.6270, 2012.
Wershaw, R. L., Friedman, I., Heller, S. J., and Frank, P. A.: Hydrogen isotopic fractionation of water passing through trees, in: Advances in Organic Geochemistry: Proceedings of the Third International Congress, edited by: Hobson, F. and Speers, M., Pergamon Press Ltd., Elsevier, New York, USA, 55–67, https://doi.org/10.1016/B978-0-08-012758-3.50007-4, 1966.
West, A. G., Patrickson, S. J., and Ehleringer, J. R.: Water extraction times for plant and soil materials used in stable isotope analysis, Rapid Commun. Mass Sp., 20, 1317–1321, https://doi.org/10.1002/rcm.2456, 2006.
White, J. W. C., Cook, E. R., Lawrence, J. R., and Wallace, S. B.: The ratios of sap in trees: Implications for water sources and tree ring ratios, Geochim. Cosmochim. Ac., 49, 237–246, https://doi.org/10.1016/0016-7037(85)90207-8, 1985.
Yang, B., Dossa, G. G. O., Hu, Y. H., Liu, L. L., Meng, X. J., Du, Y. Y., Li, J. Y., Zhu, X. A., Zhang, Y. J., Singh, A. K., Yuan, X., Wu, J. E., Zakari, S., Liu, W. J., and Song, L.: Uncorrected soil water isotopes through cryogenic vacuum distillation may lead to a false estimation on plant water source, Method. Ecol. Evol., 6, 1443–1456, https://doi.org/10.1111/2041-210X.14107, 2023.
Zhu, Q.-Z., Sun, Q., Su, Z.-G., Xie, M.-M., Song, J.-Y., Shan, Y.-B., Wang, N., and Chu, G.-Q.: A Soil Water Extraction Method with Accelerated Solvent Extraction Technique for Stable Isotope Analysis, Chinese J. Anal. Chem., 42, 1270–1275, https://doi.org/10.1016/S1872-2040(14)60766-0, 2014.