the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Understanding ecohydrology and biodiversity in aquatic nature-based solutions in urban streams and ponds through an integrative multi-tracer approach
Maria Magdalena Warter
Dörthe Tetzlaff
Chris Soulsby
Tobias Goldhammer
Daniel Gebler
Kati Vierikko
Michael T. Monaghan
Rapid urbanization and climate change affect ecohydrology, biodiversity, and water quality in urban freshwaters. Aquatic nature-based solutions (aquaNBSs) are being widely implemented to address some of the ecological and hydrological challenges that threaten urban biodiversity and water security. However, there is still a lack of process-based evidence of ecohydrological interactions in urban aquaNBSs and their relationship to water quality and quantity issues at the ecosystem level. Through a novel, integrative multi-tracer approach using stable water isotopes, hydrochemistry, and environmental DNA we sought to disentangle the effects of urbanization and hydroclimate on ecohydrological dynamics in urban aquaNBSs and understand ecohydrological functioning and the future resilience of urban freshwaters. Stable isotopes and microbial data reflected a strong influence of urban water sources (i.e., treated effluent, urban surface runoff) across stream NBSs. The results show potential limitations of aquaNBS impacts on water quality and biodiversity in effluent-impacted streams, as microbial signatures are biased towards potentially pathogenic bacteria. Urban ponds appear to be more sensitive to hydroclimate perturbations, resulting in increased microbial turnover and lower microbial diversity than expected. Furthermore, assessment of macrophytes revealed low diversity and richness of aquatic plants in both urban streams and ponds, further challenging the effectiveness of NBSs in contributing to aquatic diversity. This also demonstrates the need to adequately consider aquatic organisms in planned restoration projects, particularly those implemented in urban ecosystems, in terms of habitat requirements. Our findings emphasize the utility of integrated tracer approaches to explore the interface between ecology and hydrology and provide insights into the ecohydrologic functioning of aquaNBSs and their potential limitations. We illustrate the benefit of coupling ecological and hydrological perspectives to support future NBS design and applications that consider the interactions between water and the ecosystem more effectively.
- Article
(4516 KB) - Full-text XML
-
Supplement
(1074 KB) - BibTeX
- EndNote
Urban freshwater systems face rising anthropic and environmental pressures – from high concentrations of nutrients and other pollutants to severe hydromorphological alterations and declining streamflow, resulting in reduced biotic richness and habitat degradation (Oswald et al., 2023; Richardson and Soloviev, 2021). In addition, rapid urbanization has been a dominant paradigm and source of unintended consequences for water quality Still, urban water bodies have major potential in contributing to climate mitigation and adaptation (Bartrons et al., 2024; Hack and Schröter, 2020; van Rees et al., 2023). As a result, changing perceptions of how urban water bodies are valued has led to a surge in stream restoration measures and nature-based solutions (NBSs) as a way to address some of the ecological and societal challenges that threaten biodiversity and human well-being in urban environments (Everard and Moggridge, 2012; Fletcher et al., 2024; van Rees et al., 2023). Aquatic nature-based solutions (aquaNBSs) in particular, such as rainwater retention ponds, wetlands, or streams, are used to increase water security and reduce other water-related risks, making them key tools for urban climate mitigation and adaptation (Pinho et al., 2023).
In urban areas, aquatic nature-based solutions are often implemented to mitigate flood risk and increase water retention, enhance groundwater recharge, alleviate the urban heat island effect, and support green spaces, while also delivering major amenity and health benefits for residents (Dorst et al., 2019; Seddon et al., 2020). However, as the need for urban climate resilience continues to rise, so do concerns about the effectiveness and sustainability of NBS approaches to tackle challenges at a low cost, while delivering benefits for nature and society (Nelson et al., 2020; van Rees et al., 2023; dos Reis Oliveira et al., 2020; Seddon et al., 2020). The lack of conclusive evidence and understanding of the role of NBSs in improving climate resilience and local biodiversity, as well as their interaction with biological and hydrological conditions within water bodies, hampers a sustainable implementation of NBSs that seek to maximize the synergies while limiting trade-offs between multiple ecosystem services (Pauleit et al., 2017; Raymond et al., 2017).
Safeguarding stream biodiversity remains crucial to sustaining wider ecosystem functioning and building climate resilience under rapid change (Van der Cruysse et al., 2024). Constructed wetlands and floodplain restoration, stream reconnection, urban gardens, and re-vegetation of stream banks, as well as bio-swales or retention ponds, can help improve impaired aquatic ecosystems, targeting different ecosystem services and functions (Davis and Naumann, 2017; Dorst et al., 2019; Stefanakis, 2019). However, in urban systems, the motivation for the implementation of small-scale NBSs is often guided by simple ecological principles, such as local restoration of terrestrial and aquatic vegetation, habitat improvement, or specific local water management targets, such as water quality and flood control, while also delivering societal benefits (Van der Cruysse et al., 2024; Hack and Schröter, 2020; Hale et al., 2023). Such small-scale measures run the risk of not considering the overarching landscape-scale stressors and complex technological realities that define urbanized systems (i.e., hydrology, water chemistry, land use) (dos Reis Oliveira et al., 2020). In severely disturbed stream ecosystems, hydromorphological changes and flow management do not automatically guarantee the return and establishment of diverse ecological communities, especially if basic microbial processes, habitat, nutrient–biota relationships, and aquatic food webs are not considered (Hilderbrand et al., 2023; Leps et al., 2016).
Sustaining the benefits that come with biodiverse and resilient natural system requires healthy stream microbiomes, which include bacteria, archaea, and microalgae, as these are essential to fundamental ecosystem processes (Sehnal et al., 2021). Similarly, the adequate development of primary producers, such as large aquatic plants (macrophytes), is crucial, as they are an important component of the trophic network. Even though macrophytes may be in competition with other autotrophs, especially phytoplankton, they also provide habitat, shelter, and food for other organisms, thus significantly contributing to the overall biodiversity of aquatic ecosystems (Chambers and Maberly, 2024). The positive impact of macrophytes also extends to microbial communities, as links have been found between specific microbiomes and different habitat zones (i.e., leaf, roots, sediments, surrounding water), which are directly or indirectly provided by submerged macrophytes (Zhu et al., 2021). Similarly, relationships between microbial communities and emergent macrophyte root systems have been observed (Huang et al., 2020). Nevertheless, in urban freshwater systems, the multitude of biotic and abiotic stressors have not only led to the propagation of diverse anthropic microbial communities (McLellan et al., 2015; Vignale et al., 2023; Warter et al., 2024), but also created conditions that inherently limit macrophyte development (Gebler and Szoszkiewicz, 2022).
Given that aquatic microbes and plants are intrinsically linked to physicochemical conditions, and in particular eutrophication, they are particularly useful indicators of urban aquatic ecosystem health and functioning. Previous studies have identified macrophyte communities as reliable long-term indicators of water quality changes, incorporating changes in nutrient levels over annual (Gebler et al., 2017) or multiyear timescales (Jeppesen et al., 2005). Given their small size and rapid developmental cycles, microbial communities would be expected to respond more quickly to environmental changes. However, recent studies have shown that microbial responses to anthropogenic pressures also emerge on a seasonal or longer-term scale rather than as immediate or short-term responses (Lavoie et al., 2018; Smucker et al., 2022).
Still, evidence of increased biodiversity in NBSs is scarce. Using microbial communities, such as planktonic bacteria, benthic algae, and diatoms, in conjunction with macrophytes as integrative tracers of stream restoration and freshwater diversity can strengthen our understanding of nutrient management and restoration effectiveness. The rapid advancement of novel molecular and DNA-based approaches (i.e., environmental DNA, eDNA) now allow for targeted and efficient monitoring and evaluation of broader ecological patterns (Klaus et al., 2015; Smucker et al., 2022). A growing number of studies have used DNA-based tracers to link microbial communities to surface and subsurface flow paths (Klaus et al., 2015; Pfister et al., 2017), nutrient levels (Mansfeldt et al., 2020; Smucker et al., 2022; Zeglin, 2015), or urbanization and climate effects (Numberger et al., 2022; Warter et al., 2024). The number of studies using high-throughput sequencing of microbial communities is rapidly increasing, particularly in the urban context where higher taxonomic resolution is important to disentangle anthropogenic influences on stream aquatic diversity and the hydrological cycle (Urycki et al., 2022). Even more, in conjunction with other physical and chemical tracers, such as stable water isotopes or major ions, information on water sources and flow paths, as well as nutrient levels and pollution sources, can provide a robust tracer-based assessment of ecological, hydrological, and chemical conditions (Kuhlemann et al., 2022; Marx et al., 2021; Warter et al., 2024).
In this study, we present empirical evidence, including stable water isotopes, microbial and macrophyte richness, and diversity and hydrochemistry from established aquaNBSs in the city of Berlin, Germany. The aim of this study was to understand how urbanization, hydrology, and physiochemistry relate to microbial patterns and aquatic diversity in urban restored streams and ponds and evaluate the implications for future NBS approaches and restoration of urban freshwater systems. More specifically, our objectives were (i) to characterize the main water sources and pathways, microbial patterns, and macrophyte diversity in urban aquaNBSs, including species richness as well as alpha and beta diversity; (ii) identify the environmental drivers of microbial patterns and macrophytes; and (iii) evaluate the implications of catchment-scale stressors on aquaNBS efficiency. Our findings contribute to a broader understanding of the role of microbial processes and urban water sources in urban stream restoration and offer critical evidence to support future design and implementation of urban aquaNBSs that equally support biodiversity, ecology, and societal goals.
The city of Berlin is located in the dry NE of Germany and receives, on average, 580 mm of annual precipitation (1991–2020 mean) (DWD, 2023). Characteristic of this drier region, the majority is lost through evapotranspiration (∼56 %) (Limberg, 2007), with the remaining water distributed as urban storm drainage (12 %) and groundwater recharge (27 %). Berlin's water supply is provided through bankside filtration, involving extraction of surface water and groundwater from underlying aquifers. The majority of water is abstracted from the Spree River, which receives water from several local tributaries that enter the Spree from the north. Groundwater heads are higher near the Spree River (<4 m below ground level) and generally deeper (>10 m below ground level) on the plateaus to the north. Despite the low recharge and comparatively high evapotranspiration, Berlin has numerous wetlands and a large number of surface water bodies, including small- to medium-sized lakes and ponds (∼700) (Fig. 1c).
The central area of Berlin is highly urbanized (∼60 %), with 35 % impervious surfaces (Fig. 1d). There are also large areas of contiguous urban green space (12.1 %) and forest (17.7 %). Water quality and quantity of the major and smaller tributary rivers are characterized by land use and urban stormwater drainage, combined sewer overflows, and water abstractions (Möller and Burgschweiger, 2008). Catchment characteristics are shown in Table S1 in the Supplement.
2.1 Site description
To capture the diversity of urban freshwater systems, a range of stream and pond sites were selected (Fig. 1a). Our goal for stream sites was to choose a representative set of sequential sites downstream, experiencing varying flow conditions, urbanization effects, and surrounding land use. Furthermore, access and logistics must allow for regular sampling. Three local tributaries of the Spree, namely the Erpe, Panke, and Wuhle, were selected. Daily discharge for streams and water levels for ponds were obtained from publicly available data (SenUVK, 2024). The different streams are characterized by different land use, discharge, and restoration measures. For pond sites regular access was an important criterion for selection, along with similar size, setting (i.e., park), and permanence. The full comparison included 12 study sites: eight streams and four urban ponds. Sites were further divided into three subgroups differentiated between (i) streams with a substantial inflow of effluent from nearby wastewater treatment plants (WWTPs) and (ii) streams which do not receive effluent. This grouping was done in order to separate the effects of water sources on the physical and biological functioning of stream ecosystems (Fig. 1b). Finally, the three sample subgroups were (i) restored streams with effluent impact, (ii) restored streams without effluent impact, and (iii) restored ponds (Fig. 1b). In the following and throughout the paper, we only refer to them as streams with or without effluent impact or ponds, as all of them are restored.
2.1.1 Urban streams – with effluent impact
Erpe and Panke are both strongly impacted by treated WWTP effluent discharging from upstream treatment plants (Fig. 1a). Effluent contributions to discharge vary seasonally, ranging between 80 % during normal flows and 100 % during low-flow periods (Kuhlemann et al., 2021; Marx et al., 2021). Along both streams, successive stream restoration has been completed within the last 10 years to improve water quality and habitat conditions (SenUVK, 2009, 2013a). This included the removal of weirs, regular mowing of in-stream and bank vegetation, establishment of still-water zones, and a return to nearly natural channel structures (SenUVK, 2013b). Along the Erpe (total catchment area: 222 km2), two stream sites (Erpe HG and Erpe) in the lower catchment were selected within a 1.2 km reach. The river has a peri-urban character, with surrounding land use including agriculture and extensive wetlands (Fig. 1). Both Erpe sites are surrounded by high-density natural forest and a comparatively low degree of sealed surface, as they are in a less densely populated part of the catchment. Mean discharge is ∼ 0.8 m3 s−1 (2001–2024), which is primarily comprised of effluent discharge from the WWTP Muenchehofe (mean: 0.5 m3 s−1).
The Panke catchment (total catchment area: 220 km2) is characterized by higher urban density, but also large amounts of green space and forest, especially in the upper catchment. The three sites are located along a 6 km reach in the lower catchment. The mean discharge is ∼ 0.9 m3 s−1 (2008–2024), again with substantial effluent discharge (mean: 0.8 m3 s−1) from the WWTP Schoenerlinde (Fig. 1a). The most upstream site (Panke SP) features a meandering channel design and fish steps, as well as re-established riparian zones. At the second site (Panke OL), located within a highly urban part of the catchment, stream banks were re-vegetated and still-water zones were implemented. The third site (Suedpanke) is a side channel of the main river, where an entire stream section (∼800 m) was redesigned as part of an urban green–blue structure to improve channel flow and habitat as well as runoff retention and provide green space for residents).
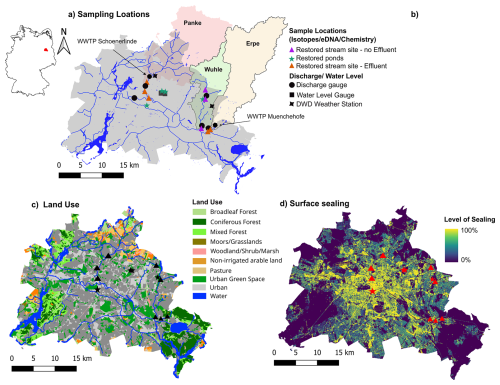
Figure 1(a) Map showing Berlin sampling sites, where stable water isotopes, hydrochemistry. and eDNA were sampled monthly between October 2022–November 2023. (b) Urban freshwater sites were grouped into three subgroups: urban ponds, urban streams with effluent impact, and urban streams without effluent impact. (c) Map of Berlin showing land use and (d) surface sealing levels, with sample sites indicated in black and red, respectively. Data source for base maps: Geoportal Berlin (Umweltatlas Berlin/ALKIS, 2022).
2.1.2 Urban streams – without effluent impact
The river Wuhle (total catchment area: 100 km2) primarily receives urban storm drainage and runoff (SenUVK, 2013b). Three sites were sampled along a 10 km river reach, with the first two (Neue Wuhle and Wuhle) located in the upper catchment within 4 km and surrounded by extensive green space. The third (Wuhle OL) is located in the more urbanized lower catchment near the stream outlet. The stream does not receive treated effluent anymore (since 2003) (SenUVK, 2013b) and has been extensively restored. The course of the river and its streambed have been previously strongly modified through straightening, deepening, and the construction of weirs to regulate flows. However, substantial efforts were undertaken to return the stream to more natural conditions and increase water retention and flood mitigation throughout the catchment. Mean discharge is ∼0.3 m3 s−1 (2002–2023), with sections of the river regularly drying out during the summer, especially in the upper catchment.
2.1.3 Urban ponds
Four representative urban ponds were included, each constructed or restored within the last 25 years. Pianosee (1.2 ha) is an entirely artificial pond within a highly urbanized setting near Potsdamer Platz in central Berlin (∼40 % sealing, <10 % green space). The pond has a maximum depth of ∼1.8 m (mean 0.5 m) and is primarily used for rainwater retention, provision of gray water for nearby office buildings, and urban cooling. Excess rainwater is stored in underground cisterns, with the majority of the water used to feed the pond. A two-stage mechanic–biologic filtration system cleans incoming rainwater and surface runoff. No water level data were available for this pond, as it is not regularly monitored. Obersee (3.7 ha) is an artificial pond situated in a natural depression. It has a maximum depth of 3 m (mean 1.49 m) and serves primarily as a retention pond and to filter surface runoff. It is situated in an urban park (16 % green space, 34 % sealed), albeit in a highly urbanized area. The pond experiences frequent water quality issues, such as phosphorus overload and excessive silt accumulation. The shore is largely lined by concrete walls, along which reed belts were constructed to support natural filtration. Orankesee (4.1 ha) is in close proximity to Obersee (distance <100 m), although the lakes are not connected. It is a natural lake fed by groundwater, with a maximum water depth of ∼6.5 m (mean 2.6 m). Half of the pond is closed off for swimming, and reed belts and still-water zones serve as habitat for water fowl. Water quality can be highly variable as the pond also receives urban runoff, but it is generally better than in Obersee due to the constant supply of freshwater. Finally, Wuhleteich (3 ha) is also an artificial pond located within a nature reserve (37 % green space, 11 % sealing) with a maximum water depth of ∼2 m (mean 0.75 m). The pond is fed by precipitation and water diverted from the nearby Wuhle. The shore is vegetated by dense reed belts and hedges, and pond substrate is very silty.
2.2 Stable water isotopes and hydrochemistry
Stable water isotopes, physicochemical parameters (pH, electrical conductivity (EC), dissolved oxygen (DO), water temperature), and solute tracers (i.e., major ions and anions) were sampled monthly from October 2022 to November 2023. For stable isotopes, water was filtered into 1.5 mL vials (LLG Labware) using 0.22 µm cellulose acetate filters. For major ions and anions, grab water samples were also filtered (0.45 µm cellulose acetate filter) before analysis. Stable water isotopes were analyzed using cavity ring-down spectroscopy with a Picarro L2130i isotopic water analyzer (Picarro Inc., Santa Clara, CA). Physicochemical parameters were measured and recorded in the field using a handheld multiprobe (Multi 3630 IDS, WTW; Weilheim, Germany). Major ions and anions were used to characterize water source end-members and flow paths. EC in particular was used as a proxy for the level of dissolved charged chemicals to determine specific water or pollution sources. Dissolved inorganic carbon (DIC), as the sum of inorganic carbon species in water, was used to characterize water quality, ecosystem health, and carbon cycling. Acid-neutralizing capacity (ANC) was also calculated using a mass balance approach of major anions and cations (Neal et al., 1999). Further details regarding sample preparation and analysis can be found in the Supplement.
Young water fractions (YWFs) were calculated using open-access code by von Freyberg et al. (2018) after Kirchner (2016), using an iteratively re-weighted least-squares (IRLS) fitted sine-wave approach. The young water fraction is defined as the fraction of water that entered a water body as recent precipitation or runoff within the last 2–3 months and is used as a metric to assess water age and transit times (von Freyberg et al., 2018; Kirchner, 2016). In the context of this study, we use YWF as a proxy for assessing residence times and hydrologic responses within urban aqua NBSs to explore links between hydrologic functioning and spatiotemporal patterns of aquatic diversity. We used observed precipitation isotopes from Berlin-Steglitz and stream and pond water isotopes to estimate the fraction of event water that entered the stream or pond within the previous 2–3 months. We compared sine-wave fit amplitudes of monthly isotope samples with amount-weighted precipitation δ18O and δ2H. To further assess evaporation effects on water isotopic conditions in streams and ponds, we used the line-conditioned excess (lc-excess), which indicates the offset of surface water samples from the local meteoric water line (LMWL) (Landwehr and Coplen, 2006). We use lc-excess as an environmental variable, in addition to water quality and hydrochemistry data, to evaluate the effect of evaporation and seasonal water loss on microbial community structures.
2.3 Microbial eDNA sequencing and analysis
Environmental DNA was collected seasonally in October 2022, January 2023, May 2024, and July 2024. Water samples (150–400 mL) were field-filtered through an Isopore polycarbonate membrane filter (Merck, Darmstadt, ø 47 mm, pore size 0.22 µm) using a hand-operated Nalgene vacuum pump and filter holder with a 500 mL receiver (both Thermo-Fisher). Filters were preserved in 2 mL Eppendorf tubes filled with 96 % ethanol and stored at −20 °C until analysis. DNA extraction and community analysis of bacteria as well as diatoms and algae were carried out on an automated workstation biomek i7 hybrid (Beckman Coulter GmbH, Krefeld, Germany) at the Berlin Center for Genomics in Biodiversity Research as described by Warter et al. (2024). Briefly, DNA was extracted using a DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol (Handbook 03/2021, HB-2495-005) with the following exceptions: in step 1, 750 µL of CD1 buffer was used, and in step 16, CD6 buffer was replaced with AE buffer from the DNeasy Blood & Tissue kit (Qiagen). AE contains EDTA in order to provide a better conservation of the samples.
Sequences were analyzed using cutadapt v 4.4 (Martin, 2013) and dada2 v 1.21.1 (Callahan et al., 2016) for R (v 4.2.0) (R Core Team, 2021). The resulting amplicon sequence variants (ASVs) were taxonomically identified using the PR2 v. 5.0.0 database (Vaulot et al., 2022) or RDP trainset 18 database (Callahan 2020). Bacteria were identified to genus level and diatoms and algae to species level. Unclassified ASVs (“NA” for species or genus level) and rare ASVs (occurring in <100 total reads or present in fewer than 10 % of the samples) were excluded from analysis to reduce the influence of sequencing artifacts.
2.4 Macrophyte sampling
An aquatic vegetation survey was carried out at each site in September 2023. Each site included two macrophyte survey sites: (i) a more natural and vegetated site, characterized by well-developed, undisturbed vegetation along the bank, and (ii) a more artificial and less vegetated site, indicating more anthropogenic disturbances. Sites were selected using a stratified random sampling approach, considering the level of naturalness and alteration. At each site, the most and least altered quadrats, spanning a 10 by 10 m square, were selected. Quadrats were then divided into a terrestrial and aquatic zone, where (i) an inventory of all species present was carried out (qualitative inventory) and (ii) species abundance was estimated (quantitative inventory). For detailed sampling design see also Szoszkiewicz et al. (2025). The survey included all groups of vascular aquatic plants, including emergent, submerged, free-floating, and floating rooted macrophytes identified at the species level. The abundance of each taxon was estimated using a nine-point level cover scale (e.g., Szoszkiewicz et al., 2020). Based on field data, species richness (N), relative abundance, and Shannon diversity were calculated for each site.
2.5 Statistical analysis
All statistical analyses were performed using R Studio (version 4.2.3) (R Core Team, 2021). The vegan package was used to assess ecological data (Oksanen, 2022). Spatial and seasonal differences in physiochemical parameters and hydrochemistry were identified using Kruskal–Wallis testing (α=0.05). Assessments of microbial community patterns were performed using relative abundance data. Standard methods for the representation of multidimensional data were used, such as nonmetric multidimensional scaling (NMDS), for the evaluation of spatial patterns in microbial diversity using the Bray–Curtis dissimilarity index (Dexter et al., 2018). Community structures were assessed through pairwise comparison analysis of similarities (ANOSIM). To characterize microbial α diversity, genus and species richness (i.e., total number of species present) was calculated for bacteria as well as diatoms and algae, respectively. We used the Shannon diversity (Shannon H index), which takes into account the relative abundance of different species rather than just species richness, to evaluate the overall diversity of species in each subgroup (Hill et al., 2003). Differences in alpha diversity for microbial and macrophyte communities between subgroups were evaluated through nonparametric Kruskal–Wallis tests (p value of <0.05) and mixed two-way analysis of variance (ANOVA) (Wang et al., 2016).
Taxonomic β diversity was assessed using the betapart package in R (Baselga and Orme, 2012), using relative abundance data transformed into binary presence or absence data (presence: 1, absence: 0). The Sorensen dissimilarity index was used for all pairwise combinations of assemblages between sample subgroups (n=3) based on presence or absence data. Multivariate dispersion was then calculated using the Jaccard dissimilarity index, which gauges the similarity and diversity of sample sets (Real and Vargas, 1996). To summarize the variation in community structure among subgroups, homogeneity was assessed based on the mean distance of samples to their corresponding group centroid (“distance to centroid”) (Anderson et al., 2006). Statistical differences in mean centroid distances between sample groups were tested using PERMANOVA, using the adonis function in the vegan package, with 999 permutations. Distance-based redundancy analysis (dbRDA) was finally used to explore the relationship between environmental parameters and relative abundance (Legendre and Andersson, 1999).
3.1 Stable water isotopes
Stable water isotopes illustrated the differences in water sources and flow paths between ponds and streams (Fig. 2). The evaporation line was well below the GMWL and the LMWL. Isotopic signatures from ponds confirmed evaporative fractionation, which was also reflected in the more depleted lc-excess values, particularly in summer and autumn. In comparison, effluent-impacted streams showed limited variability and more depleted values, with moderately negative lc-excess as a result of concurrent mixing of multiple water sources, including local groundwater, surface runoff, and effluent discharge (Kuhlemann et al., 2020, 2021; Marx et al., 2021, 2023). Streams with no effluent discharge showed more variability, with seasonally more depleted signatures and overall more enriched lc-excess.
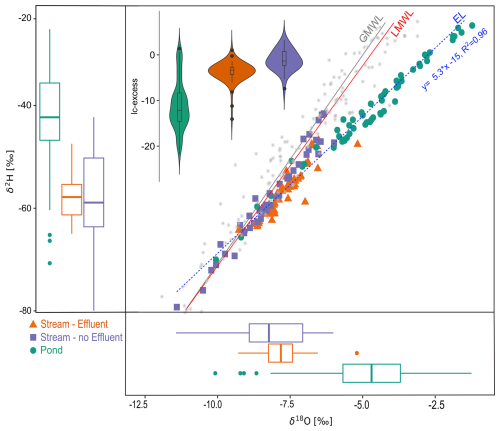
Figure 2Dual isotope plot (center) and box plots (left, bottom) showing the isotopic composition of the different surface water types, as well as lc-excess (inset). In box plots, the horizontal line indicates the mean, and whiskers indicate the range of values from minimum to maximum. Outliers are indicated as dots. The global meteoric water line (GMWL, gray), amount-weighted local meteoric water line (LMLW, red) from precipitation samples in Berlin-Steglitz, and evaporation line (EL, blue) are given for reference.
Estimates of young water fractions (YWFs; percent of water younger than 2–3 months) in ponds were high, ranging between 50 % and >90 % (Table 1). YWFs in the effluent-impacted Panke and Erpe were characteristically lower (∼10 %) due to the dampening influence of wastewater (see also Fig. S1). However, there was some variability in YWF estimates from samples of the Panke River, with estimates of up to 50 % (RSE = 0.5) in the most downstream restored section (Suedpanke), which is highly influenced by weir management and urban surface runoff. YWF from the non-effluent-impacted Wuhle ranged from 40 % in the lower catchment to >90 % in the upper catchment, with greater uncertainties in the upper catchment (RSE ∼1.0). This is in line with the variable influence and response of streamflow to seasonal precipitation and surface runoff, as well as groundwater in the upper and lower catchment, respectively.
3.2 Hydrochemistry
Across all stream and pond sites, pH was slightly alkaline with means around 7.9 (SD = 0.6; Fig. S1, Table S2). EC varied distinctly between sample groups (p<0.001), with higher EC in effluent-impacted streams. Between ponds, EC also varied significantly, with higher EC observed in Obersee (Fig. S2, Table S2). The highest DIC concentrations were observed in the lower catchment sites of the non-effluent-impacted Wuhle (up to 85 mg L−1) but on average were highest in the effluent-impacted Erpe and Panke and lowest in ponds. Dissolved oxygen in surface water varied significantly between subgroups (p=0.02), reflecting seasonal differences in local biological productivity. DO levels were highest in ponds and lowest in the non-effluent-impacted Wuhle in autumn and summer. Water temperatures ranged between 19–23 °C in summer and 1.5–6.8 °C in winter.
Clear differences in major ion sources between stream and pond sites reflected the tributary nutrient loads from effluent discharge into streams and the influence of dominant water sources (e.g., GW, surface runoff, precipitation) in ponds and non-effluent-impacted streams also seen in the isotopic signatures (see also Fig. S3). Most notably in the effluent-impacted Erpe and Panke, levels of nitrate nitrogen (NO3N), SO4, Ca, K, Mg, P, S, and Si were much higher compared to non-effluent-impacted streams and ponds (see Table S3). Concentrations of Na and Cl were on average the highest in effluent-impacted streams, but also in Obersee. Elevated concentrations of Ca were observed in effluent-impacted streams, as well as in Obersee (see Fig. S3), which can be attributed to GW contributions from local aquifers, which tend to be Ca-rich in Berlin. Of all ponds, Obersee also showed higher levels of S and SO4. Other trace metals such as Cu, Fe, Mn, and Zn levels were negligible in all samples (<0.001 mg L−1). ANC was highest in effluent-impacted streams due to overall increased nutrient loads. ANC showed large variability in the non-effluent-impacted Wuhle and ponds, likely as a result of varying source water contributions (i.e., urban runoff, GW), also evident in the more variable stable isotope signatures.
3.3 Microbial community characterization
Of the 14 034 bacterial ASVs, 48 % (total 6693) could be assigned to a genus, ∼80 % of which (total 5465) were present in >10 % of the samples. The final dataset of bacteria consisted of 42 samples, with a total of 212 genera and a mean of 83 genera in each sample (range: 31–161). For diatoms and algae, 7125 ASVs were obtained, 65 % of which (total 4626) could be assigned to a species and of those ∼48 % (total 2188) were detected in >10 % of the samples. The final dataset of diatoms and algae consisted of 46 samples, with a mean of 89 genera per sample (range: 5–162). NMDS ordination of microbial communities shows an acceptable representation (stress <0.2 for both) (Fig. 3). The community structures of bacteria as well as diatoms and algae were significantly different according to the NMDS (both p=0.001), with communities of ponds being clearly differentiated from streams. Significant differences between stream and pond sites were confirmed by pairwise comparison of similarities (ANOSIM, r=0.51, p=0.001) for bacteria as well as diatoms and algae (ANOSIM, r=0.46, p=0.001). Microbial communities in effluent and non-effluent-impacted streams appear closely clustered in the ordination plot. However, the pairwise comparison indicated significant differences between effluent and non-effluent-impacted streams in bacteria (ANOSIM: r=0.53, p=0.001) as well as diatom and algae communities (ANOSIM: r=0.35, p=0.001). Seasonal differences in bacterial assemblages were significant in effluent-impacted streams (p=0.001) but negligible in other subgroups. For diatoms and algae, samples of streams visibly converged in all seasons, suggesting similar seasonal community assemblage, although pairwise comparison (ANOSIM, r=0.31, p=0.001) indicates some distinction between seasonal samples.
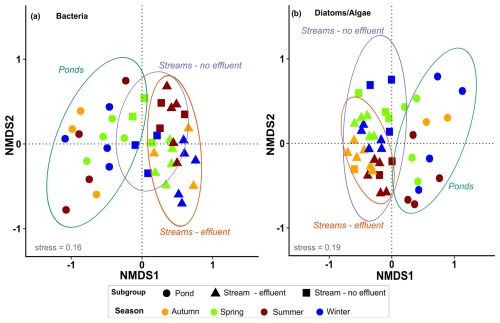
Figure 3Nonmetric multidimensional scaling using the Bray–Curtis dissimilarity index for (a) bacteria (genus level, stress: 0.16) and (b) diatoms and algae (species level, stress: 0.19) in urban streams (with and without effluent) and ponds. Seasonal samples are indicated by colors, and shapes denote different subgroups. Ellipses indicate the 95 % confidence interval for each subgroup.
3.4 Alpha diversity
Compared to stream sites (mean: ∼110), about ∼50 % fewer bacterial genera were found in ponds (mean: 55). Pairwise comparisons of genus richness indicated no difference among stream subgroups (p=0.72) but between pond and stream subgroups (p<0.001) (Fig. 4a). Most notably, in certain ponds and the non-effluent-impacted Wuhle, there was a high relative abundance of Cyanobacteria (up to 35 %). In ponds, they were most abundant in spring and lowest in autumn, while in the Wuhle they were most abundant in winter. Shannon diversity was not significantly different between ponds and effluent-impacted streams (p=0.12), while diversity in the non-effluent-impacted Wuhle clearly differed from ponds and the effluent-impacted Erpe and Panke (Fig. 4d).
Diatom and algae species richness differed significantly among all sample subgroups (p=0.004) (Fig. 4b), with fewer species in ponds (mean: 75) compared to streams (mean: 103). Post hoc pairwise comparison revealed significant differences between ponds and the effluent-impacted Wuhle (p<0.001). Shannon diversity was similar (p=0.2) in all subgroups, suggesting no significant divergence in species diversity (Fig. 4e). Although the composition of diatom and algae communities in streams appeared significant according to ANOSIM, the similarities in Shannon diversity suggest limited variation in species composition.
Macrophyte richness was low in all streams (Fig. 4c). Overall species richness did not show significant variability between subgroups across levels of naturalness (all p>0.1). Species richness was lowest in the non-effluent-impacted Wuhle, with the identified taxa indicative of more eutrophic conditions, such as Phalaris arundinacea, Iris pseudoacorus, Persicaria hydropiper, and Sparganium emersum (see Table S4 in the Supplement for a complete list of species). These species represent emergent macrophytes (helophyte vegetation), which develop well in shallow zones of stagnant or slow-flowing waters and are also characteristic for nutrient-rich water or sediments. The highest species richness was observed in the effluent-impacted Panke (n=12). Also, emergent macrophytes were dominant in the Panke, but beyond them, a greater diversity of submerged vegetation was recorded, with most of the species specific to nutrient-rich waters. Shannon diversity also showed no significant variability between subgroups, except in the non-effluent-impacted Wuhle (p=0.01) (Fig. 4f). However, this group only consists of two sample sites and thus may not be fully representative.
Pond sites exhibited greater consistency and comparable levels of macrophyte diversity. The diversity indices obtained from ponds were significantly higher than those from effluent-impacted streams yet lower than those from the non-effluent-impacted Wuhle. Additionally, the variability among individual ponds was comparatively lower. The findings indicated that, regardless of whether the sites were categorized as ponds, effluent, or non-effluent streams, the more anthropic locations were characterized by increased biodiversity. This phenomenon was less attributable to the anthropogenic alterations of the aquatic ecosystems and more related to the underdeveloped riparian or terrestrial vegetation linked to the transformation of the bank zone. Consequently, these modifications resulted in improved light availability (reduced shading), fostering enhanced growth and diversity of macrophytes.
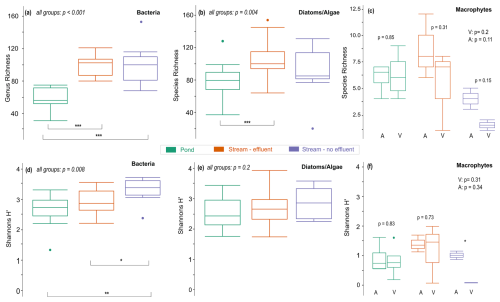
Figure 4(a) Genus richness for bacteria, (b) species richness for diatoms and algae, and (c) species richness for macrophytes. (d–f) Shannon diversity for bacteria, diatoms and algae, and macrophytes. Horizontal lines in the box plots represent median values, and dots indicate outliers. Coloring corresponds to sample subgroups. P values denote significant differences in alpha diversity measured across all sample groups or between two individual groups. Statistical significance between two subgroups is indicated with asterisks at the 0.05 (*), 0.01 (**), and 0.001 (***) level. Macrophyte sampling sites are differentiated between vegetated (V) and artificial (A) sites.
3.5 Beta diversity
Beta diversity dispersion, considering Bray–Curtis dissimilarity, varied between all sample groups for bacteria (F=2.91, p=0.001) as well as diatoms and algae (F=3.02, p=0.01) (Fig. 5). Overall, bacterial community dissimilarity was highest in ponds (0.41) compared to effluent and non-effluent-impacted streams (0.30 and 0.28, respectively). Similarly, β diversity of diatoms and algae was highest in ponds (0.51) compared to streams (0.45 in effluent-impacted, 0.47 in non-effluent-impacted) (see also Fig. S5). Considering all possible pairs for comparison between stream and pond sites, microbial dissimilarity was significant only between ponds and streams but not between the two stream subgroups (bacteria: p=0.96; diatoms and algae: 0.19), which contrasts with the dissimilarity observed in the NMDS (Fig. 3). Partitioning total beta diversity into the relevant components, bacterial seasonal turnover (βSIM) was highest for ponds (0.37) and lowest in the non-effluent-impacted Wuhle (0.16), (see also Table S5). Similarly, diatom seasonal turnover was also highest in restored ponds (0.49) and lowest in the non-effluent-impacted Wuhle (0.29).
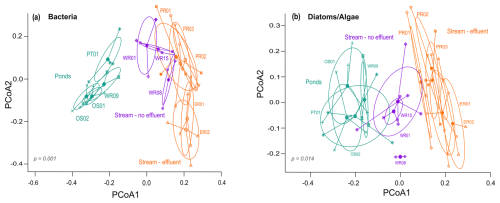
Figure 5Multivariate beta diversity dispersion of bacteria as well as diatoms and algae. Beta diversity is measured as the distance of samples to their group centroid (using Jaccard distances), shown here as the first two axes of a PCoA. On the PCoA, a change in site dispersion around the centroid indicates a change in beta diversity, while a change in the centroid location indicates species turnover. Symbols represent each stream or pond site, and colors indicate the overall grouping.
3.6 Influence of environmental variables on microbial and macrophyte communities
Distance-based RDA revealed that almost 50 % (R2=0.69, ) of the variation of bacteria in streams and ponds (R2=0.83, ) could be explained by physicochemical and hydrological parameters (see Table S6 for a summary of RDA model results). The environmental variables most significant for the bacteria–environment relationship in streams were discharge (p=0.001), water temperature (p=0.001), DIC (p=0.007), DO (p=0.004), percentage of green space (p=0.003), YWF (p=0.005), Na (p=0.04), B (p=0.04), and EC (p=0.01) (Fig. 6a). Opposite relationships of bacterial community assemblage with nutrients and discharge variability separated bacterial communities between effluent- and non-effluent-impacted streams. YWF was positively correlated in the non-effluent-impacted Wuhle, where YWFs were generally higher than in the effluent-impacted Panke and Erpe (see also Table 1). Statistically, the first two RDA axes were significant (dbRDA1: p=0.001; dbRDA2: p=0.007), cumulatively explaining 38 % of the total variation, with most of the variation occurring along the first axis (22.52 %). Along axis 2, water temperature was a key driver of seasonal changes of bacterial communities, broadly separating summer and spring from winter and autumn samples, especially in the effluent-impacted streams.
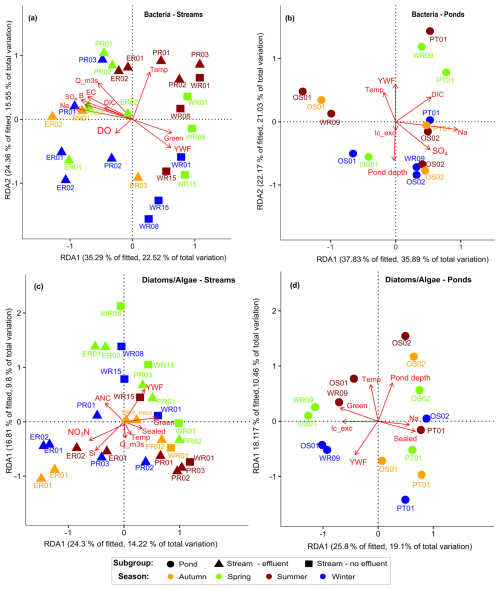
Figure 6Distance-based redundancy analysis (dbRDA) plot of a distance-based linear model (DBLM) of key environmental parameters fitted to the variation in bacteria as well as diatoms and algae for (a, c) streams (effluent- and non-effluent-impacted) and (b, d) ponds. Arrows represent the direction of a parameter effect, colors indicate sampling seasons, and symbols denote subgroups for streams and pond sites.
In ponds, pond depth (p=0.013), water temperature (p=0.019), DIC (p=0.001), YWF (p=0.003), Na (p=0.01), SO4 (p=0.03), and lc-excess (p=0.01) were most significant (Fig. 6b). The first two axes were significant (dbRDA1: p=0.003: dbRDA2: 0.03), with ∼36 % of the variation occurring along the first axis (Fig. 6b). Na, DIC, and SO4 were positively correlated with bacterial assemblage in ponds PT01 and OS02, in line with increased surface runoff reaching these ponds. YWF, water temperature, lc-excess, and pond depth broadly separated seasonal samples, indicating a seasonal sensitivity to changing water levels and hydroclimate conditions, in line with the highly variable water isotope signatures and evaporative fractionation observed in ponds.
For diatoms and algae up to 30 % of the variation in communities (R2=0.61, ) in streams and ∼20 % in ponds (R2=0.66, ) could be constrained through surrounding land use or hydrologic and physicochemical parameters. In streams, YWF (p=0.001), percentage of green space (p=0.001), percentage of sealed surfaces (p=0.009), discharge (p=0.004), NO3N (p=0.01), water temperature (p=0.003), ANC (p=0.002), and Si (p=0.03) (Fig. 6c) were most significant. The parameters that significantly constrained diatom communities were the percent of sealed surfaces (p=0.004), pond depth (p=0.005), Na (p=0.04), and YWF (p=0.002).
Due to only one sample date, pond and stream macrophytes were not divided by season or locality. Multivariate RDA showed that up to 50 % of macrophyte diversity (R2=0.73, ) could be explained by concentrations of NO3N (p=0.001), P (p=0.004), Ca (p=0.001), alkalinity (p=0.008), pH (p=0.002), DO (p=0.007), YWF (p=0.01), and water levels (0.001). The results broadly match the patterns observed in microbial communities, with nutrient-rich waters in effluent-impacted streams accommodating specific species not found in the other sites. Compared to streams, the generally higher young water contributions and higher DO levels in ponds appear to contribute to the similar levels of macrophyte diversity observed across all ponds. The exception was OS-01, which had a high abundance of Iris pseudoacorus L. (swamp iris) and dense reed beds (Typa angustifolia L.), which were less dominant or absent in the other ponds. Water level fluctuations appeared positively correlated with species diversity in ponds but negatively in streams.
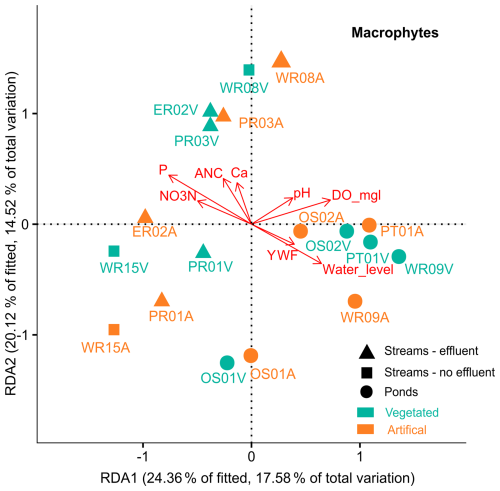
Figure 7Distance-based redundancy analysis (dbRDA) plot of a DBLM (distance based linear model) of key environmental parameters fitted to the variation in the relative abundance of macrophytes for streams and ponds. Symbols denote sample groups, and colors denote artificialness (green – vegetated, orange – artificial). Arrows represent the direction of a parameter effect, colors indicate sampling seasons, and symbols denote subgroups for streams and pond sites.
4.1 Differences in microbial and macrophyte diversity
This study provides an integrated evaluation of the chemical and hydrological functioning of contrasting urban aquatic nature-based solutions. The observed differences in microbial diversity illustrate the influence of effluent on community composition in the Erpe and Panke throughout the stream continuum (see also Kuhlemann et al., 2021; Marx et al., 2021; Warter et al., 2024). Multiple studies have already tied the effects of rapid urbanization and increasing connectivity between urban water bodies and sewer lines to changes in natural freshwater bacterial communities across urban streams (Lee et al., 2020; McLellan et al., 2015; Numberger et al., 2022; Vignale et al., 2023). In cases where surface waters receive water from different urban sources, such as urban storm drains and effluent discharge, their good ecological status remains constantly compromised due to the increased influx of nutrients and harmful substances (i.e., antibiotics, pharmaceuticals, etc.) (Büttner et al., 2022; Lee et al., 2020; Reid et al., 2019). This is most evident in the abundance of highly specific bacterial communities associated with the human digestive system (i.e., Romboutsia, Intestinibacter, Clostridium), as well as N- and P-tolerant diatoms (i.e., Nitzschia amphibia, Polytoma uvella), throughout the whole downstream environment of the effluent-impacted Erpe and Panke. Similarly, the high abundance of Cyanobacteria during summer and autumn in the non-effluent-impacted Wuhle was likely a result of more eutrophic conditions, propagated by high urban runoff after storm events. In both cases, hydrochemistry, eDNA, and stable water isotopes confirm the overarching fingerprint of urban-specific water sources on ecologic and hydrologic functioning. Based on our results, we would argue that the present ecological and hydrological conditions suggest a certain mismatch between the initial restoration goals and the current state of the surveyed stream sections. Overall, the findings build on a previous study by Warter et al. (2024) in Berlin, confirming that the extensive influence of wastewater essentially limits the ecological potential, favoring the abundance of more nutrient-tolerant species, as seen in diatom as well as macrophyte species. The higher richness of macrophyte species in the effluent-dominated Erpe and Panke suggests that in urban environments, where organisms are affected by multiple pressures, the effluent input may be an important factor favoring the development of aquatic plants that prefer or thrive in high concentrations of nutrients (Gebler and Szoszkiewicz, 2022; Silva et al., 2020). This may create additional beneficial contributions to the purification of water from nutrients (Levi et al., 2015; Toerien and Toerien, 1985). Aquatic plants can also be an important element in shaping microbial communities, which is related not only to specific species of macrophytes (Wang et al., 2024), but also to their functional traits and diversity (Özbay, 2018; Zhu et al., 2021). Specific functional groups of microbes may also be associated with hydrophyte litter decomposition, which may further promote the development of microbial communities associated with reducing nutrient loads (Kumwimba et al., 2023; Toerien and Toerien, 1985). Still, the higher richness in effluent-impacted streams and the fact that bacteria show a more pronounced influence from effluent discharge than diatoms and algae may warrant further examination, particularly of sediment communities and composition as they constitute another reservoir of organic and inorganic compounds over time (Haller et al., 2009; Huang et al., 2020; Wang et al., 2023).
While nutrient levels and hydrologic variability were effective indicators of microbial and macrophyte communities in streams, pinpointing the key factors of microbial variability in ponds was more difficult. The observed low alpha diversity contained within urban ponds was at first surprising. This was generally in line with previous studies, which noted low biodiversity in urban ponds, including aquatic plants, macroinvertebrates, and amphibians, due to the influence of water quality and urban landscape structure (Hamer and Parris, 2011; Oertli et al., 2023; Oertli and Parris, 2019). As the number of plant and animal species inhabiting a pond is strongly impacted by the surrounding land use, a more urban matrix tends to limit the dispersal or exchange of species, often leading to biologic isolation of certain ponds (Oertli and Parris, 2019). Similarly, the exposure to urban water sources (i.e., storm runoff) and water level fluctuations can create variable conditions that can promote greater species turnover (Siddha and Sahu, 2022), which is line with the observed high beta diversity and turnover in sampled ponds. The high evaporative enrichment also suggests a certain sensitivity to hydroclimate, with purely rainfed ponds such as Pianosee, Wuhleteich, and Obersee at risk of experiencing disproportionate water losses during hot and dry periods.
As urban ponds are often designed with predominately social or aesthetic ecosystem services in mind, the promotion and conservation of local native biodiversity through suitable, if less aesthetic, habitat often fall behind (Bartrons et al., 2024; Cuenca-Cambronero et al., 2023; Oertli and Parris, 2019). The resulting biotic homogenization, due to a desire for uniform environmental conditions and functional designs, thus creates habitat niches that will generally benefit only a limited number of species (Hassall, 2014; Numberger et al., 2022). Beside physicochemical conditions, an observed lack of natural substrates and suitable plant beds and the presence of steep concrete shorelines likely limited the establishment of more diverse aquatic species and macrophytes in observed ponds.
4.2 Future resilience of urban aquaNBSs
Our study provides a novel inventory of hydrological and ecological functioning of aquaNBSs within urban streams and ponds, while simultaneously challenging some of the restoration measures that were undertaken and their actual contribution to aquatic diversity. The disparity between the perceived effectiveness of restoration and the actual impact on aquatic diversity and habitat quality still presents a major issue for restoration efforts, especially in urban areas. This is due to a continued lack of post hoc assessment of restoration objectives and a significant knowledge gap in maximizing blue infrastructures as effective nature-based solutions (Bartrons et al., 2024; Hilderbrand et al., 2023; Oertli and Parris, 2019; dos Reis Oliveira et al., 2020). To date, macroinvertebrate assessments are the most common methods used for assessing aquatic diversity (Durance and Ormerod, 2009; Fergus et al., 2023). However, challenges in the level of detection, taxonomic assignment, sampling technique, and representativeness make it less viable for applications over extended temporal and spatial scales. As such, the multi-tracer approach constitutes a novel and innovative alternative to streamline assessments and long-term monitoring of in-stream restoration and associated effects on hydrological and ecological functioning.
Based on our results, we argue that to select appropriate aquaNBSs and avoid ecosystem disservices (Lyytimäki and Sipilä, 2009), it is essential to first identify the current and most relevant combinations of stressors that affect ecological conditions of streams – i.e., increased nutrients, urban runoff, and surrounding land use. For this, the integrated application of environmental tracers, such as stable isotopes and eDNA, has proven useful in quantifying the biophysical interactions needed to characterize ecosystem functioning, which in turn can help to implement appropriate restoration measures. As the movement of water, matter, and organisms, as well as interactions with the surrounding landscape, is substantially different in urbanized systems (Oswald et al., 2023), prioritizing the scales on which stream restoration measures should have a measurable impact (local, regional, or watershed) is critical for the viability of the NBS. Especially for aquatic nature-based solutions, upstream processes influence downstream conditions and local hydrology, and the potential effects of hydroclimate changes will dictate the functioning and long-term benefits of the NBS. As such, water managers need to account for how the NBS might evolve over time with ongoing climate change and changing water availability.
Secondly, in riverine ecosystems it is crucial not to disregard natural system complexity in favor of simplistic and static minimum flow designations, especially considering the effects of climate change and urban stormwater (Acreman et al., 2014; Arthington et al., 2006). In the case of the non-effluent-impacted Wuhle, an increasing trend towards intermittency in recent years and a more variable flow regime coincide with an observed limited macrophyte diversity, lower water quality, and high abundance of cyanobacteria. This suggests that past restoration measures may not be able to counteract the ongoing loss of hydrological connectivity, making it unlikely to improve ecological conditions in the future. As such, projections of longer droughts followed by intense rainfall are likely to reduce the efficacy of NBSs that depend on vegetation–surface water interactions to improve water quality and streamflow regimes (Fletcher et al., 2024). In drought-prone regions, effluent discharge has already become an increasingly important water source for sustaining such declining streamflow regimes (Luthy et al., 2015), stabilizing discharge variability, and keeping baseflows high even in times of drought (Lawrence et al., 2014; Plumlee et al., 2012). However, there are noted caveats to consider for aquatic habitats, with potentially long-term effects on ecology, chemistry, and flow dynamics, which are still not well established (Büttner et al., 2022). As an alternative, integrating aquaNBSs in the form of restored wetlands or enhanced river channel restoration, to engage hyporheic filtration processes or slow-rate soil infiltration, would more actively support the removal of pollutants in effluent before they are discharged into stream ecosystems (Büttner et al., 2022; Cross et al., 2021; Stefanakis, 2019).
Due to the pervasive influence of urbanization on water quality in the studied ponds, the contributions to aquatic diversity ultimately appears to be quite limited. Indeed, a caveat to this conclusion is that we did not consider terrestrial vegetation or higher-order aquatic organisms, such as amphibians, macroinvertebrates, or fish, as this was outside the scope of this study. However, diversity patterns of bacteria as well as diatom and algae communities already provided an initial assessment of ecological conditions, which could be clearly linked to hydrology, water quality, and urban influences. This is not to say that urban water bodies cannot support freshwater biodiversity at all or provide stormwater storage, nutrient processing, or recreation opportunities (Fletcher et al., 2024; Hassall, 2014). Indeed, there are many examples of threatened and endangered species finding refuge in urban parks, wetlands and ponds. Examples include populations of macroinvertebrates (Hill et al., 2016), damselflies and dragonflies (Goertzen and Suhling, 2015), and amphibians (Holtmann et al., 2017). However, the proximity to human activities, limited green space, and increasing exposure to urbanization and climate effects have clearly affected microbial diversity in the studied ponds. Here we argue that pond design must consider not only physical requirements but also the physiochemical habitat quality and biological functions in order to reconcile ecologic with aesthetic or social ecosystem services (Bartrons et al., 2024).
In the future, to improve the success and longevity of restoration projects, clear expectations need to be set for the provision of ecosystem services and desired contributions to biodiversity (Bartrons et al., 2024; Oertli and Parris, 2019). The focus on trade-offs between societal and environmental issues, such as flood control, recreation, or carbon storage, often leaves ecological and biodiversity aspects behind or considered separately, when in fact they are deeply connected and often share the same drivers (Seddon et al., 2020). More importantly, expectations may differ between protecting relatively unimpacted natural streams and restoring already degraded streams. For example, restoration goals in heavily degraded streams may aim simply create ecosystem structure and functions that provide certain services and social benefits (i.e., recreation, rainwater retention) rather than completely restoring natural conditions (Fletcher et al., 2024). Such highly modified urban water bodies may then still support water storage, nutrient processing, and recreation or the provision of space and amenities for residents but fail to significantly contribute to biodiversity (Seddon et al., 2020).
Despite certain caveats to urban stream restoration and urban aquatic nature-based solutions, well-designed approaches that match relevant biophysical and ecological objectives and restoration goals by considering basic microbial processes, physiochemistry, and hydrological functioning of aquatic environments, as well as relevant spatiotemporal scales, can greatly support climate change mitigation while also providing key benefits to people and the natural environment. Due to the important functions they perform, macrophytes can be a vital element in restoring and shaping the appropriate structure of aquatic ecosystems (Suren, 2009). This can also increase the diversity of all organisms associated with its occurrence and the general diversity of freshwaters (Fu et al., 2024; Paice et al., 2016). However, to achieve positive ecological outcomes, including enhanced water quality and rehabilitation of native populations, site-specific management and restoration strategies tailored to biotic and abiotic conditions (i.e., habitat, streamflow permanence) are crucial.
Our analysis of urban aquaNBSs in streams and ponds within the city of Berlin demonstrated the influence of anthropogenic and environmental stressors on hydrologic variability, water chemistry, and microbial diversity. Despite the extensive restoration measures undertaken in all streams within the last decade, urbanization remains a multifaceted driver that impacts aquaNBSs. Our use of an integrated multi-tracer approach proved to be highly insightful in qualitatively assessing microbial and macrophyte diversity and relationships with environmental parameters, addressing questions related to the effectiveness of restoration measures and aquaNBSs in contributing to local aquatic diversity. Our research has shown that (i) microbial and macrophyte diversity is heavily influenced by urban water sources, and ecological potential may be severely limited in effluent-impacted streams; (ii) microbial diversity was lowest in urban ponds, with a high abundance of Cyanobacteria and low water quality; (iii) a high abundance of Cyanobacteria and low macrophyte richness and diversity in non-effluent-impacted streams suggest low contributions to local aquatic diversity despite extensive restoration; (iv) microbial processes should be considered in future restoration efforts to create balanced ecosystems and improve the ecological status of degraded ecosystems; and (v) the still limited research on restoration effects on biodiversity warrants further research to enhance the climate resilience of urban water bodies.
Data will be made available on request. Metadata can be found on the Freshwater Research and Ecology Database (FRED) at https://doi.org/10.18728/igb-fred-939.0 (Warter, 2024). Climate data were publicly available from the German Weather Service (https://opendata.dwd.de/climate_environment/CDC/observations_germany/climate/hourly/precipitation/, DWD, 2023). Stream discharge and water levels are publicly available from the Berlin Senate (https://wasserportal.berlin.de/start.php, SenUVK, 2024).
The supplement related to this article is available online at https://doi.org/10.5194/hess-29-2707-2025-supplement.
Conceptualization: DT, CS, MM. Methodology: MMW, DT, CS, MTM, TG, DG. Investigation: MMW, DG. Formal analysis: MMW, DG. Data curation: MMW, DG. Writing (original draft): MMW. Writing (review and editing): DT, CS, MTM, TG, KV, DG. Visualization: MMW. Supervision: DT, CS. Funding acquisition: DT, MTM, KV.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thankfully acknowledge Franziska Schmidt from the IGB Isotope Lab for the isotope analysis. We are also very grateful to colleagues from the Chemical Analytics and Biogeochemistry Lab of IGB for water chemistry analysis, in particular Claudia Schmalsch, Thomas Rossoll, and Marvin Sens. For support with the metabarcoding analysis of eDNA samples we thankfully acknowledge Sarah Sparmann, Susan Mbedi, and Stephanie Neitzel. We also thank Jan Christopher for help with sampling.
This study was funded through BiodivRestore for the Binatur project (BMBF no. 16WL015). Dörthe Tetzlaff also received funding through the Einstein Research Unit “Climate and Water under Change” from the Einstein Foundation Berlin and Berlin University Alliance (grant no. ERU-2020-609) and through the German Research Foundation (DFG) as part of the Research Training Group “Urban Water Interfaces” (UWI, grant no. GRK2032/2). Research was also partially funded by the Einstein Stiftung Berlin, MOSAIC project (grant no. EVF-2018-425). Michael T. Monaghan received funding from the German Federal Ministry of Education and Research (BMBF, grant no. 033W034A). Daniel Gebler was also funded through the Binatur project, which is partially financed by the National Science Centre (Poland) (grant no. UMO-2021/03/Y/NZ8/00100).
This paper was edited by Julia Knapp and reviewed by two anonymous referees.
Acreman, M., Arthington, A. H., Colloff, M. J., Couch, C., Crossman, N. D., Dyer, F., Overton, I., Pollino, C. A., Stewardson, M. J., and Young, W.: Environmental flows for natural, hybrid, and novel riverine ecosystems in a changing world, Front. Ecol. Environ., 12, 466–473, https://doi.org/10.1890/130134, 2014.
Anderson, M. J., Ellingsen, K. E., and McArdle, B. H.: Multivariate dispersion as a measure of beta diversity, Ecol. Lett., 9, 683–693, https://doi.org/10.1111/j.1461-0248.2006.00926.x, 2006.
Arthington, A. H., Bunn, S. E., Poff, N. L. R., and Naiman, R. J.: The challenge of providing environmental flow rules to sustain river ecosystems, Ecol. Appl., 16, 1311–1318, https://doi.org/10.1890/1051-0761(2006)016[1311:TCOPEF]2.0.CO;2, 2006.
Bartrons, M., Trochine, C., Blicharska, M., Oertli, B., Lago, M., and Brucet, S.: Unlocking the potential of ponds and pondscapes as nature-based solutions for climate resilience and beyond: Hundred evidences, J. Environ. Manage., 359, 120992, https://doi.org/10.1016/j.jenvman.2024.120992, 2024.
Baselga, A. and Orme, C. D. L.: Betapart: An R package for the study of beta diversity, Methods Ecol. Evol., 3, 808–812, https://doi.org/10.1111/j.2041-210X.2012.00224.x, 2012.
Büttner, O., Jawitz, J. W., Birk, S., and Borchardt, D.: Why wastewater treatment fails to protect stream ecosystems in Europe, Water Res., 217, 118382, https://doi.org/10.1016/j.watres.2022.118382, 2022.
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., and Johnson, A.: DADA2: high-resolution sample inference from Illumina amplicon data, Nat. Methods, 3, 581–583, https://doi.org/10.1038/nmeth.3869, 2016.
Chambers, P. A. and Maberly, S. C.: Chapter 24 - Freshwater Plants, in: Wetzel's Limnology (Fourth Edition), edited by: Jones, I. D. and Smol, J. P., Academic Press, San Diego, 759–816, https://doi.org/10.1016/B978-0-12-822701-5.00024-0, 2024.
Cross, K., Tondera, K., Rizzo, A., Andrews, L., Pucher, B., Istenič, D., Karres, N., and Mcdonald, R.: Solutions for Solutions Nature-Based Solutions Wastewater Treatment for Wastewater for Wastewater, 344 pp., IWA Publishing, London, ISBN 9781789062250, 2021.
Cuenca-Cambronero, M., Blicharska, M., Perrin, J. A., Davidson, T. A., Oertli, B., Lago, M., Beklioglu, M., Meerhoff, M., Arim, M., Teixeira, J., De Meester, L., Biggs, J., Robin, J., Martin, B., Greaves, H. M., Sayer, C. D., Lemmens, P., Boix, D., Mehner, T., Bartrons, M., and Brucet, S.: Challenges and opportunities in the use of ponds and pondscapes as Nature-based Solutions, Hydrobiologia, 850, 3257–3271, https://doi.org/10.1007/s10750-023-05149-y, 2023.
Davis, M. and Naumann, S.: Making the Case for Sustainable Urban Drainage Systems as a Nature-Based Solution to Urban Flooding, 123–137 pp., https://doi.org/10.1007/978-3-319-56091-5_8, 2017.
Dexter, E., Rollwagen-Bollens, G., and Bollens, S. M.: The trouble with stress: A flexible method for the evaluation of nonmetric multidimensional scaling, Limnol. Oceanogr. Methods, 16, 434–443, https://doi.org/10.1002/lom3.10257, 2018.
Dorst, H., van der Jagt, A., Raven, R., and Runhaar, H.: Urban greening through nature-based solutions – Key characteristics of an emerging concept, Sustain. Cities Soc., 49, 101620, https://doi.org/10.1016/j.scs.2019.101620, 2019.
dos Reis Oliveira, P. C., van der Geest, H. G., Kraak, M. H. S., Westveer, J. J., Verdonschot, R. C. M., and Verdonschot, P. F. M.: Over forty years of lowland stream restoration: Lessons learned?, J. Environ. Manage., 264, 110417, https://doi.org/10.1016/j.jenvman.2020.110417, 2020.
Durance, I. and Ormerod, S. J.: Trends in water quality and discharge confound long-term warming effects on river macroinvertebrates, Freshw. Biol., 54, 388–405, https://doi.org/10.1111/j.1365-2427.2008.02112.x, 2009.
DWD (Deutscher Wetterdienst): Climate data center (CDC), Deutscher Wetterdienst [data set], https://opendata.dwd.de/climate_environment/CDC/observations_germany/climate/hourly/precipitation/ (last access: 14 June 2024), 2022.
Everard, M. and Moggridge, H. L.: Rediscovering the value of urban rivers, Urban Ecosyst., 15, 293–314, https://doi.org/10.1007/s11252-011-0174-7, 2012.
Fergus, C. E., Brooks, J. R., Kaufmann, P. R., Herlihy, A. T., Hill, R. A., Mitchell, R. M., and Ringold, P.: Disentangling natural and anthropogenic effects on benthic macroinvertebrate assemblages in western US streams, Ecosphere, 14, 1–24, https://doi.org/10.1002/ecs2.4688, 2023.
Fletcher, T. D., Burns, M. J., Russell, K. L., Hamel, P., Duchesne, S., Cherqui, F., and Roy, A. H.: Concepts and evolution of urban hydrology, Nat. Rev. Earth Environ., 5, 789–801, https://doi.org/10.1038/s43017-024-00599-x, 2024.
Fu, H., Xu, J., García Molinos, J., Zhang, H., Wang, H., Zhang, M., Klaar, M., and Brown, L. E.: Macroinvertebrate and environmental responses to dredging and submerged macrophytes transplantation, J. Appl. Ecol., 61, 1041–1052, https://doi.org/10.1111/1365-2664.14620, 2024.
Gebler, D. and Szoszkiewicz, K.: Response of Aquatic Plants to Extreme Alterations in River Morphology, Water, 14, 3746, https://doi.org/10.3390/w14223746, 2022.
Gebler, D., Szoszkiewicz, K., and Pietruczuk, K.: Modeling of the river ecological status with macrophytes using artificial neural networks, Limnologica, 65, 46–54, https://doi.org/10.1016/j.limno.2017.07.004, 2017.
Goertzen, D. and Suhling, F.: Central European cities maintain substantial dragonfly species richness – a chance for biodiversity conservation?, Insect Conserv. Divers., 8, 238–246, https://doi.org/10.1111/icad.12102, 2015.
Hack, J. and Schröter, B.: Nature-Based Solutions for River Restoration in Metropolitan Areas, Palgrave Encycl. Urban Reg. Futur., Springer Nature Link, https://doi.org/10.1007/978-3-030-51812-7, 2020.
Hale, S. E., von der Tann, L., Rebelo, A. J., Esler, K. J., de Lima, A. P. M., Rodrigues, A. F., Latawiec, A. E., Ramírez-Agudelo, N. A., Bosch, E. R., Suleiman, L., Singh, N., and Oen, A. M. P.: Evaluating Nature-Based Solutions for Water Management in Peri-Urban Areas, Water (Switzerland), 15, 1–34, https://doi.org/10.3390/w15050893, 2023.
Haller, L., Amedegnato, E., Poté, J., and Wildi, W.: Influence of Freshwater Sediment Characteristics on Persistence of Fecal Indicator Bacteria, Water. Air. Soil Pollut., 203, 217–227, https://doi.org/10.1007/s11270-009-0005-0, 2009.
Hamer, A. J. and Parris, K. M.: Local and landscape determinants of amphibian communities in urban ponds, Ecol. Appl., 21, 378–390, https://doi.org/10.1890/10-0390.1, 2011.
Hassall, C.: The ecology and biodiversity of urban ponds, Wiley Interdiscip. Rev. Water, 1, 187–206, https://doi.org/10.1002/wat2.1014, 2014.
Hilderbrand, R. H., Bambakidis, T., and Crump, B. C.: The Roles of Microbes in Stream Restorations, Microb. Ecol., 85, 853–861, https://doi.org/10.1007/s00248-023-02179-w, 2023.
Hill, M. J., Ryves, D. B., White, J. C., and Wood, P. J.: Macroinvertebrate diversity in urban and rural ponds: Implications for freshwater biodiversity conservation, Biol. Conserv., 201, 50–59, https://doi.org/10.1016/j.biocon.2016.06.027, 2016.
Hill, T. C. J., Walsh, K. A., Harris, J. A., and Moffett, B. F.: Using ecological diversity measures with bacterial communities, FEMS Microbiol. Ecol., 43, 1–11, https://doi.org/10.1016/S0168-6496(02)00449-X, 2003.
Holtmann, L., Philipp, K., Becke, C., and Fartmann, T.: Effects of habitat and landscape quality on amphibian assemblages of urban stormwater ponds, Urban Ecosyst., 20, 1249–1259, https://doi.org/10.1007/s11252-017-0677-y, 2017.
Huang, R., Zeng, J., Zhao, D., Cook, K. V., Hambright, K. D., and Yu, Z.: Sediment microbiomes associated with the rhizosphere of emergent macrophytes in a shallow, subtropical lake, Limnol. Oceanogr., 65, S38–S48, https://doi.org/10.1002/lno.11325, 2020.
Jeppesen, E., Søndergaard, M., Jensen, J. P., Havens, K. E., Anneville, O., Carvalho, L., Coveney, M. F., Deneke, R., Dokulil, M. T., Foy, B., Gerdeaux, D., Hampton, S. E., Hilt, S., Kangur, K., Köhler, J., Lammens, E. H. H. R., Lauridsen, T. L., Manca, M., Miracle, M. R., Moss, B., Nõges, P., Persson, G., Phillips, G., Portielje, R., Romo, S., Schelske, C. L., Straile, D., Tatrai, I., Willén, E., and Winder, M.: Lake responses to reduced nutrient loading – An analysis of contemporary long-term data from 35 case studies, Freshw. Biol., 50, 1747–1771, https://doi.org/10.1111/j.1365-2427.2005.01415.x, 2005.
Kirchner, J. W.: Aggregation in environmental systems – Part 1: Seasonal tracer cycles quantify young water fractions, but not mean transit times, in spatially heterogeneous catchments, Hydrol. Earth Syst. Sci., 20, 279–297, https://doi.org/10.5194/hess-20-279-2016, 2016.
Klaus, J., Wetzel, C. E., Martínez-Carreras, N., Ector, L., and Pfister, L.: A tracer to bridge the scales: On the value of diatoms for tracing fast flow path connectivity from headwaters to meso-scale catchments, Hydrol. Process., 29, 5275–5289, https://doi.org/10.1002/hyp.10628, 2015.
Kuhlemann, L. M., Tetzlaff, D., and Soulsby, C.: Urban water systems under climate stress: An isotopic perspective from Berlin, Germany, Hydrol. Process., 34, 3758–3776, https://doi.org/10.1002/hyp.13850, 2020.
Kuhlemann, L. M., Tetzlaff, D., and Soulsby, C.: Spatio-temporal variations in stable isotopes in peri-urban catchments: A preliminary assessment of potential and challenges in assessing streamflow sources, J. Hydrol., 600, 126685, https://doi.org/10.1016/j.jhydrol.2021.126685, 2021.
Kuhlemann, L. M., Tetzlaff, D., Marx, C., and Soulsby, C.: The imprint of hydroclimate, urbanization and catchment connectivity on the stable isotope dynamics of a large river in Berlin, Germany, J. Hydrol., 613, https://doi.org/10.1016/j.jhydrol.2022.128335, 2022.
Kumwimba, M. N., Dzakpasu, M., Li, X., Huang, J., Ajibade, F. O., Muyembe, D. K., and Mihiranga, H. K. M.: Vegetated urban streams have sufficient purification ability but high internal nutrient loadings: Microbial communities and nutrient release dynamics, Sci. Total Environ., 863, 160921, https://doi.org/10.1016/j.scitotenv.2022.160921, 2023.
Landwehr, J. M. and Coplen, T. .: Line-conditioned excess: a new method for characterizing stable hydrogen and oxygen isotope ratios in hydrologic systems (IAEA-CN-118/56), in: Isotopes in Environmental Studies, IAEA, Vienna, 132–135, 2006.
Lavoie, I., Morin, S., Laderriere, V., and Fortin, C.: Freshwater Diatoms as Indicators of Combined Long-Term Mining and Urban Stressors in Junction Creek (Ontario, Canada), Environments, 5, 30, https://doi.org/10.3390/environments5020030, 2018.
Lawrence, J. E., Pavia, C. P. W., Kaing, S., Bischel, H. N., Luthy, R. G., and Resh, V. H.: Recycled water for augmenting urban streams in mediterranean-climate regions: a potential appraoch for riparian ecosystem enhancement, Hydrol. Sci. J., 59, 488–501, https://doi.org/10.1080/02626667.2013.818221, 2014.
Lee, S., Suits, M., Wituszynski, D., Winston, R., Martin, J., and Lee, J.: Residential urban stormwater runoff: A comprehensive profile of microbiome and antibiotic resistance, Sci. Total Environ., 723, 138033, https://doi.org/10.1016/j.scitotenv.2020.138033, 2020.
Legendre, P. and Andersson, M. J.: Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments, Ecol. Monogr., 69, 1–24, https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2, 1999.
Leps, M., Sundermann, A., Tonkin, J. D., Lorenz, A. W., and Haase, P.: Time is no healer: Increasing restoration age does not lead to improved benthic invertebrate communities in restored river reaches, Sci. Total Environ., 557–558, 722–732, https://doi.org/10.1016/j.scitotenv.2016.03.120, 2016.
Levi, P. S., Riis, T., Alnøe, A. B., Peipoch, M., Maetzke, K., Bruus, C., and Baattrup-Pedersen, A.: Macrophyte complexity controls nutrient uptake in lowland streams, Ecosystems, 18, 914–931, https://doi.org/10.1007/s10021-015-9872-y, 2015.
Limberg, A.: Grundwasser in Berlin. Vorkommen-Nutzung-Schutz-Gefährdung, Berlin/Senatsverwaltung für Gesundheit, Umwelt und Verbraucherschutz, Berlin, URN: urn:nbn:de:kobv:109-opus-133492, 2007.
Luthy, R. G., Sedlak, D. L., Plumlee, M. H., Austin, D., and Resh, V. H.: Wastewater-effluent-dominated streams as ecosystem-management tools in a drier climate, Front. Ecol. Environ., 13, 477–485, https://doi.org/10.1890/150038, 2015.
Lyytimäki, J. and Sipilä, M.: Hopping on one leg – The challenge of ecosystem disservices for urban green management, Urban For. Urban Green., 8, 309–315, https://doi.org/10.1016/j.ufug.2009.09.003, 2009.
Mansfeldt, C., Deiner, K., Mächler, E., Fenner, K., Eggen, R. I. L., Stamm, C., Schönenberger, U., Walser, J.-C., and Altermatt, F.: Microbial community shifts in streams receiving treated wastewater effluent, Sci. Total Environ., 709, 135727, https://doi.org/10.1016/j.scitotenv.2019.135727, 2020.
Martin, M.: Cutadapt removes adapter sequences from high-throughput sequencing reads, Bioinforma. action, 17, 10–12, https://doi.org/10.14806/ej.17.1.200, 2013.
Marx, C., Tetzlaff, D., Hinkelmann, R., and Soulsby, C.: Isotope hydrology and water sources in a heavily urbanized stream, Hydrol. Process., 35, 1–20, https://doi.org/10.1002/hyp.14377, 2021.
Marx, C., Tetzlaff, D., Hinkelmann, R., and Soulsby, C.: Effects of 66 years of water management and hydroclimatic change on the urban hydrology and water quality of the Panke catchment, Berlin, Germany, Sci. Total Environ., 900, 165764, https://doi.org/10.1016/j.scitotenv.2023.165764, 2023.
McLellan, S. L., Fisher, J. C., and Newton, R. J.: The microbiome of urban waters, Int. Microbiol., 18, 141–149, https://doi.org/10.2436/20.1501.01.244, 2015.
Möller, K. and Burgschweiger, J.: Wasserversorgungskonzept für Berlin und für das von den BWB versorgte Umland (Entwicklung bis 2040), vol. 85, 1–85, Umweltvorhaben Dr. Klaus Möller GmbH, Berlin, 2008.
Neal, C., Reynolds, B., and Robson, A. J.: Acid neutralisation capacity measurements within natural waters: Towards a standardised approach, Sci. Total Environ., 243–244, 233–241, https://doi.org/10.1016/S0048-9697(99)00385-X, 1999.
Nelson, D. R., Bledsoe, B. P., Ferreira, S., and Nibbelink, N. P.: Challenges to realizing the potential of nature-based solutions, Curr. Opin. Environ. Sustain., 45, 49–55, https://doi.org/10.1016/j.cosust.2020.09.001, 2020.
Numberger, D., Zoccarato, L., Woodhouse, J., Ganzert, L., Sauer, S., Márquez, J. R. G., Domisch, S., Grossart, H. P., and Greenwood, A. D.: Urbanization promotes specific bacteria in freshwater microbiomes including potential pathogens, Sci. Total Environ., 845, 157321, https://doi.org/10.1016/j.scitotenv.2022.157321, 2022.
Oertli, B. and Parris, K. M.: Review: Toward management of urban ponds for freshwater biodiversity, Ecosphere, 10, e02810, https://doi.org/10.1002/ecs2.2810, 2019.
Oertli, B., Decrey, M., Demierre, E., Fahy, J. C., Gallinelli, P., Vasco, F., and Ilg, C.: Ornamental ponds as Nature-based Solutions to implement in cities, Sci. Total Environ., 888, 164300, https://doi.org/10.1016/j.scitotenv.2023.164300, 2023.
Oksanen, J.: _vegan: Community Ecology Package_. R package version 2.6–4, R Foundation for Statistical Computing, Vienna, https://github.com/vegandevs/vegan (last access: 24 March 2024), 2022.
Oswald, C. J., Kelleher, C., Ledford, S. H., Hopkins, K. G., Sytsma, A., Tetzlaff, D., Toran, L., and Voter, C.: Integrating urban water fluxes and moving beyond impervious surface cover: A review, J. Hydrol., 618, 129188, https://doi.org/10.1016/j.jhydrol.2023.129188, 2023.
Özbay, H.: Bacterial community on submersed plants in running water, Desalin. Water Treat., 106, 267–272, https://doi.org/10.5004/dwt.2018.22078, 2018.
Paice, R. L., Chambers, J. M., and Robson, B. J.: Outcomes of submerged macrophyte restoration in a shallow impounded, eutrophic river, Hydrobiologia, 778, 179–192, https://doi.org/10.1007/s10750-015-2441-8, 2016.
Pauleit, S., Zölch, T., Hansen, R., Randrup, T. B., Konijnendijk, van den and Bosch, C. K.: Chapter 3: Nature-based solutions and climate change – four shades of green, in: Nature-based solutions to climate change adaptation in urban areas: Linkages between Science, Policy and Practice, edited by: Kabisch, N., Korn, H., Stadler J., and Bonn, A., 29–51, https://doi.org/10.1007/978-3-319-56091-5, 2017.
Pfister, L., Wetzel, C. E., Klaus, J., Martínez-Carreras, N., Antonelli, M., Teuling, A. J., and McDonnell, J. J.: Terrestrial diatoms as tracers in catchment hydrology: a review, Wiley Interdiscip. Rev. Water, 4, 1–13, https://doi.org/10.1002/WAT2.1241, 2017.
Pinho, P., Haase, D., Gebler, D., Staes, J., Martelo, J., Schoelynck, J., Szoszkiewicz, K., Monaghan, M. T., and Vierikko, K.: Urban Aquatic Nature-Based Solutions in the Context of Global Change: Uncovering the Social-ecological-technological Framework, 139–157, https://doi.org/10.1007/978-3-031-34378-0_8, 2023.
Plumlee, M. H., Gurr, C. J., and Reinhard, M.: Recycled water for stream flow augmentation: Benefits, challenges, and the presence of wastewater-derived organic compounds, Sci. Total Environ., 438, 541–548, https://doi.org/10.1016/j.scitotenv.2012.08.062, 2012.
Raymond, C. M., Frantzeskaki, N., Kabisch, N., Berry, P., Breil, M., Nita, M. R., Geneletti, D., and Calfapietra, C.: A framework for assessing and implementing the co-benefits of nature-based solutions in urban areas, Environ. Sci. Policy, 77, 15–24, https://doi.org/10.1016/j.envsci.2017.07.008, 2017.
R Core Team: R: A language and environment for statistical computing, https://www.r-project.org/ (last access: 1 March 2024), 2021.
Real, R. and Vargas, J. M.: The Probabilistic Basis of Jaccard's Index of Similarity, Syst. Biol., 45, 380–385, https://doi.org/10.2307/2413572, 1996.
Reid, A. J., Carlson, A. K., Creed, I. F., Eliason, E. J., Gell, P. A., Johnson, P. T. J., Kidd, K. A., MacCormack, T. J., Olden, J. D., Ormerod, S. J., Smol, J. P., Taylor, W. W., Tockner, K., Vermaire, J. C., Dudgeon, D., and Cooke, S. J.: Emerging threats and persistent conservation challenges for freshwater biodiversity, Biol. Rev., 94, 849–873, https://doi.org/10.1111/brv.12480, 2019.
Richardson, M. and Soloviev, M.: The urban river syndrome: Achieving sustainability against a backdrop of accelerating change, Int. J. Environ. Res. Public Health, 18, 6406, https://doi.org/10.3390/ijerph18126406, 2021.
Seddon, N., Chausson, A., Berry, P., Girardin, C. A. J., Smith, A., and Turner, B.: Understanding the value and limits of nature-based solutions to climate change and other global challenges, Philos. T. Roy. Soc. B, 375, https://doi.org/10.1098/rstb.2019.0120, 2020.
Sehnal, L., Brammer-Robbins, E., Wormington, A. M., Blaha, L., Bisesi, J., Larkin, I., Martyniuk, C. J., Simonin, M., and Adamovsky, O.: Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health, Front. Microbiol., 12, 567408, https://doi.org/10.3389/fmicb.2021.567408, 2021.
SenUVK (Senate Department for Environment, Transport and Climate Protection): Panke 2015: Ein Bach wird naturnah, Berlin Senatsverwaltung für Gesundheit, Umwelt und Verbraucherschutz, 2009.
SenUVK (Senate Department for Environment, Transport and Climate Protection): Ökologische Entwicklung der Erpe: Informationsheft zur europäischen Wasserrahmenrichtline (WRRL), Berlin Senatsverwaltung für Stadtentwicklung und Umwelt, 2013a.
SenUVK (Senate Department for Environment, Transport and Climate Protection): Ökologische Entwicklung der Wuhle: Informationsheft zur europäischen Wasserrahmenrichtlinie (WRRL), Berlin Senatsverwaltung für Stadtentwicklung und Umwelt, 2013b.
SenUVK (Senate Department for Environment, Transport and Climate Protection): Wasserportal – Gewässerkundliche Messdaten, https://wasserportal.berlin.de/start.php (14 June 2024), 2024.
Siddha, S. and Sahu, P.: Chapter 5 – Impact of climate change on the river ecosystem, in: Ecological Significance of River Ecosystems, edited by: Madhav, S., Kanhaiya, S., Srivastav, A., Singh, V., and Singh, P., Elsevier, 79–104, https://doi.org/10.1016/B978-0-323-85045-2.00014-5, 2022.
Silva, F. L. da, Stefani, M. S., Smith, W. S., Schiavone, D. C., da Cunha-Santino, M. B., and Bianchini, I.: An applied ecological approach for the assessment of anthropogenic disturbances in urban wetlands and the contributor river, Ecol. Complex., 43, 100852, https://doi.org/10.1016/j.ecocom.2020.100852, 2020.
Smucker, N. J., Pilgrim, E. M., Wu, H., Nietch, C. T., Darling, J. A., Molina, M., Johnson, B. R., and Yuan, L. L.: Characterizing temporal variability in streams supports nutrient indicator development using diatom and bacterial DNA metabarcoding, Sci. Total Environ., 831, 154960, https://doi.org/10.1016/j.scitotenv.2022.154960, 2022.
Stefanakis, A. I.: The Role of Constructed Wetlands as Green Infrastructure for Sustainable Urban Water Management, Sustain., 11, 6981, https://doi.org/10.3390/su11246981, 2019.
Suren, A. M.: Using macrophytes in urban stream rehabilitation: A cautionary tale, Restor. Ecol., 17, 873–883, https://doi.org/10.1111/j.1526-100X.2008.00446.x, 2009.
Szoszkiewicz, K., Jusik, S., Pietruczuk, K., and Gebler, D.: The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions, Water, 12, 108, https://doi.org/10.3390/w12010108, 2020.
Szoszkiewicz, K., Achtenberg, K., Debbaut, R., Carreira, V. D., Gebler, D., Jusik, S., Kałuża, T., Karttunen, K., Lehti, N., Muñoz, S. M., Sojka, M., Pereira, A. J., Pinho, P., Schoelynck, J., Staes, J., Tetzlaff, D., Warter, M. M., and Vierikko, K.: Diversification of macrophytes within aquatic nature-based solutions (NBS) developing under urban environmental conditions across European cities, Ecol. Indic., 172, 113331, https://doi.org/10.1016/j.ecolind.2025.113331, 2025.
Toerien, D. F. and Toerien, M. C.: Microbial heterotrophy in an effluent treatment system using macrophytes, Agric. Wastes, 12, 287–312, https://doi.org/10.1016/0141-4607(85)90027-7, 1985.
Umweltatlas Berlin/ALKIS: FIS Broker, FIS broker, https://fbinter.stadt-berlin.de/fb/index.jsp (last access: 12 March 2022), 2022.
Urycki, D. R., Bassiouni, M., Good, S. P., Crump, B. C., and Li, B.: The streamwater microbiome encodes hydrologic data across scales, Sci. Total Environ., 849, 157911, https://doi.org/10.1016/j.scitotenv.2022.157911, 2022.
Van der Cruysse, L., De Cock, A., Lock, K., Boets, P., and Goethals, P. L. M.: Introduction of Native Submerged Macrophytes to Restore Biodiversity in Streams, Plants, 13, https://doi.org/10.3390/plants13071014, 2024.
van Rees, C. B., Jumani, S., Abera, L., Rack, L., McKay, S. K., and Wenger, S. J.: The potential for nature-based solutions to combat the freshwater biodiversity crisis, PLOS Water, 2, e0000126, https://doi.org/10.1371/journal.pwat.0000126, 2023.
Vaulot, D., Geisen, S., Mahé, F., and Bass, D.: pr2-primers: An 18S rRNA primer database for protists, Molec. Ecol. Resour., 22, 168–179, https://doi.org/10.1111/1755-0998.13465, 2022.
Vignale, F. A., Bernal Rey, D., Pardo, A. M., Almasqué, F. J., Ibarra, J. G., Fernández Do Porto, D., Turjanski, A. G., López, N. I., Helman, R. J. M., and Raiger Iustman, L. J.: Spatial and Seasonal Variations in the Bacterial Community of an Anthropogenic Impacted Urban Stream, Microb. Ecol., 85, 862–874, https://doi.org/10.1007/s00248-022-02055-z, 2023.
von Freyberg, J., Allen, S. T., Seeger, S., Weiler, M., and Kirchner, J. W.: Sensitivity of young water fractions to hydro-climatic forcing and landscape properties across 22 Swiss catchments, Hydrol. Earth Syst. Sci., 22, 3841–3861, https://doi.org/10.5194/hess-22-3841-2018, 2018.
Wang, H., Liu, X., Wang, Y., Zhang, S., Zhang, G., Han, Y., Li, M., and Liu, L.: Spatial and temporal dynamics of microbial community composition and factors influencing the surface water and sediments of urban rivers, J. Environ. Sci. (China), 124, 187–197, https://doi.org/10.1016/j.jes.2021.10.016, 2023.
Wang, P., Chen, B., Yuan, R., Li, C., and Li, Y.: Characteristics of aquatic bacterial community and the influencing factors in an urban river, Sci. Total Environ., 569–570, 382–389, https://doi.org/10.1016/j.scitotenv.2016.06.130, 2016.
Wang, X., Liu, Y., Qing, C., Zeng, J., Dong, J., and Xia, P.: Analysis of diversity and function of epiphytic bacterial communities associated with macrophytes using a metagenomic approach, Microb. Ecol., 87, 37, https://doi.org/10.1007/s00248-024-02346-7, 2024.
Warter, M.: BiNatur - Biodiversity in urban Rivers, IGB Leibniz-Institute of Freshwater Ecology and Inland Fisheries [data set], https://doi.org/10.18728/igb-fred-939.0, 2024.
Warter, M. M., Tetzlaff, D., Ring, A. M., Christopher, J., Kissener, H. L., Funke, E., Sparmann, S., Mbedi, S., Soulsby, C., and Monaghan, M. T.: Environmental DNA, hydrochemistry and stable water isotopes as integrative tracers of urban ecohydrology, Water Res., 250, 121065, https://doi.org/10.1016/j.watres.2023.121065, 2024.
Zeglin, L. H.: Stream microbial diversity in response to environmental changes: Review and synthesis of existing research, Front. Microbiol., 6, 1–15, https://doi.org/10.3389/fmicb.2015.00454, 2015.
Zhu, H. Z., Jiang, M. Z., Zhou, N., Jiang, C. Y., and Liu, S. J.: Submerged macrophytes recruit unique microbial communities and drive functional zonation in an aquatic system, Appl. Microbiol. Biotechnol., 105, 7517–7528, https://doi.org/10.1007/s00253-021-11565-8, 2021.