the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Technical note: GUARD – an automated fluid sampler preventing sample alteration by contamination, evaporation and gas exchange, suitable for remote areas and harsh conditions
Arno Hartmann
Marc Luetscher
Ralf Wachter
Philipp Holz
Elisabeth Eiche
Thomas Neumann
Automated water sampling devices adapted to field operation have proven highly useful for environmental research as well as in the public and private sectors, where natural or artificial waters need to be tested regularly for compliance with environmental and health regulations. Such autosamplers are already available on the market in slightly differing versions, but none of these devices are capable of sealing the collected samples to prevent sample alteration by contamination, evaporation or gas exchange. In many sampling cases, however, this feature is essential, for instance for studying the hydrological cycle based on isotopes in rainwater, or for monitoring waters contaminated with toxic gases or other volatile compounds detrimental to biota and human health. Therefore, we have developed a new mobile autosampler, which injects water samples directly into airtight vials, thus preventing any sample alteration. Further advantages include low production costs, compact dimensions and low weight allowing for easy transport, a wide range of selectable sampling intervals as well as a low power consumption, which make it suitable for long-term applications even in remote areas and harsh (outdoor) conditions due to its heavy-duty water-proof casing. In this paper, we demonstrate (1) the sampler's mechanical functioning, (2) the long-term stability of the collected samples with regard to evaporation and gas exchange and (3) the potential of our device in a wide variety of applications drawing on laboratory and field experiments in different karst caves, which represent one of the most challenging sampling environments.
- Article
(3196 KB) - Full-text XML
-
Supplement
(837 KB) - BibTeX
- EndNote
Sampling natural waters (water bodies and rainwater), as well as artificial waters (including process and waste water) for subsequent hydrochemical and geochemical analysis, is indispensable for understanding the behaviour of natural and artificial waters, for determining their spatial and temporal variability (e.g. in the concentration of toxic compounds) as well as for determining their current state (e.g. Hiscock and Bense, 2014). A thorough monitoring of water is thus important, not only for scientific purposes, but also to prevent adverse effects on human health and ecosystems, whether in the short, medium or long term.
However, the manual collection of water samples is not only time-consuming, but also expensive and logistically challenging. This is particularly true in remote areas with poor or no infrastructure, where expenses for field trips and equipment transport to the sampling site and back are often significantly increased. Furthermore, in most cases the properties and composition of the water under investigation change with time, for instance, the concentration of pollutants like heavy metals or polycyclic aromatics (Appelo and Postma, 2005). In these cases, it is necessary to repeat water sampling multiple times at short intervals and over a sufficiently long time period, increasing the logistical constraints (e.g. Ahuja, 2013). When it comes to long-term monitoring of a site at relatively short intervals, manual sampling is definitely not a viable option anymore (Chapin, 2015).
This impasse can only be overcome by automation: autosamplers suitable for field operation, such as the 3700C Compact portable sampler (Teledyne ISCO, USA), are already available on the market and offer the opportunity to automatically and repeatedly sample water bodies (oceans, estuaries, lakes, rivers, groundwater, etc.). As they can be powered by batteries or solar panels, these autosamplers do not need a connection to the grid and can, therefore, be applied even in remote areas.
Despite their suitability for a wide range of applications, available autosamplers lack the capacity to automatically seal the sample vials after collection. This, however, is absolutely essential in many sampling applications where any exchange of gases and/or volatile components between the sample and the ambient air needs to be prevented in order to preserve the sample's original properties until analysis. Examples are numerous and include cases of water being contaminated by toxic gases originating from anaerobic degradation of organics (e.g. CH4 and H2) or by volatile organic compounds (VOCs), such as tetrachloroethene, benzene, MTBE or formaldehyde (e.g. Reemtsma and Jekel, 2010).
Sample vials also need to be gastight directly after sample collection where evaporation or condensation has to be impeded to prevent sample alteration, for example where non-volatile components like cations and anions need to be quantified. In such cases evaporation would cause erroneously augmented concentration values (Hiscock and Bense, 2014), especially in long-term monitoring schemes.
Similarly, the prevention of evaporation is paramount in all studies investigating the hydrological cycle based on the stable isotopes of hydrogen and oxygen (indicated as δD and δ18O values). The majority of such studies rely on rainwater samples (mostly) collected manually at stations of the Global Network of Isotopes in Precipitation (GNIP; IAEA/WMO, 1994) coordinated by the International Atomic Energy Agency (IAEA) with the sampling performed by dedicated partner institutions in member states of the IAEA or the World Meteorological Organisation (WMO). At these stations, rainwater is generally sampled at monthly resolution to ensure worldwide compatibility of GNIP data from different sources. While most of these samples are collected manually, a number of active or passive totalizers compliant with the GNIP sampling guidelines (Terzer et al., 2016) are in operation at GNIP stations without permanent staffing. Manual sampling at higher temporal resolution, such as rainfall event-based sampling, is practically impossible as this would require round-the-clock stand-by duty.
Furthermore, sample alteration due to evaporation is commonly prevented by sealing the water samples' surface with paraffin oil despite it causing an increased need for maintenance of the standard instrument for water isotope analysis, i.e. cavity ring-down spectroscopy (CRDS).
Establishing an isotope baseline for meteoric waters is crucial for research in hydrology, meteorology and other scientific fields. While remarkable progress has been made thanks to GNIP data, the network is still spatially and temporally discontinuous, among other reasons due to the practical constraints on rainwater sampling in remote areas. Automated rainwater sampling could help solve this issue and increased maintenance of spectrometers could be avoided by applying gastight sample vials. This clearly illustrates the need for automated rainwater sampling with gastight sample vials.
Furthermore, the need for automated liquid sampling in general is demonstrated by a number of technical developments by multiple groups with the aim of creating automated liquid samplers capable of sealing the samples after collection. For instance, researchers at Oregon State University have developed the “OPEnSampler” (Nelke et al., 2017; http://www.open-sensing.org/opensampler/, last access: 30 June 2018) that comprises an array of 24 solenoid valves, allowing the 24 sampling containers to be sealed from the environment after sample collection. Lukas Neuhaus has developed the “Lisa Liquidsampler” (not published) that fills 48 sample vials sealed by septa (engineered membranes that permit the transfer of fluids without air contact, usually using a double canula) using a vacuum pump via 48 separate transfer tubes. Applying a new automated precipitation collector obtaining 96 sequential 15 mL samples, Coplen et al. (2008) were able to measure a strong decrease of 51 % in the hydrogen isotope ratio (δD) of precipitation over only 1 h resulting from the landfall of an extratropical cyclone along the coast of California. Evaporation and subsequent isotopic fractionation were minimised by a Teflon-coated vial cover; thus, sample vials are not sealed individually.
In addition to these newly developed liquid autosamplers that are (1) suited for field operation in remote areas and under harsh (outdoor) conditions and (2) capable of sealing the sample vials (gastight) directly after sample collection, we have designed, constructed and tested a new autosampler (“GUARD”) that also fulfils these requirements, but that can be equipped with up to 160 sample vials due to its space-efficient design. In addition to fulfilling the former requirements, the GUARD autosampler offers further advantages including a low weight, low cost (∼ USD 1000), compact dimensions, easy transport, a wide range of selectable sampling intervals as well as a low power consumption, which permits either high-frequency sampling (e.g. every minute), long-term monitoring (e.g. 6 months), or medium-term monitoring at medium sampling frequency (e.g. daily sampling for 48 days). Therefore, the GUARD autosampler is applicable for a wide spectrum of sampling purposes, ranging from studies on characterization of high-frequency variabilities to long-term changes in natural or artificial waters. By sealing the sample vials with septa (engineered membranes that permit the transfer of fluids without air contact, usually using a double canula), not only undesired gas exchange and evaporation/condensation are prevented, but also any contamination that might otherwise occur, especially in long-term monitoring projects where sample vials may need to stay at the sampling site for several months or longer. This protection is further ensured by a water-tight and airtight sampler case, which prevents damage from extreme weather conditions (e.g. water or dust ingress, high humidity) and also protects the samples from any external interference, e.g. from animal activity.
The set-up and design of the GUARD autosampler are described in Sect. 2. In Sect. 3 we draw on test runs in the “laboratory” and in a karst cave, one of the most challenging sampling environments, to demonstrate (1) the mechanical functioning and (2) the long-term chemical stability of the collected samples. In Sect. 4 we demonstrate the potential of our autosampler in a wide variety of applications by presenting the results of a 5-day case study at a sampling interval of only 4 h, which would have been hardly possible without the use of our device. In Sect. 5 we compare our invention to already existing autosamplers suitable for field operation and conclude by highlighting potential sampling applications.
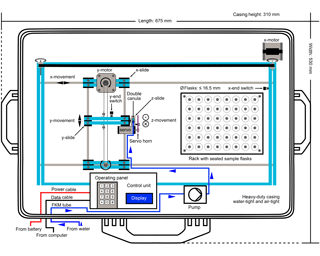
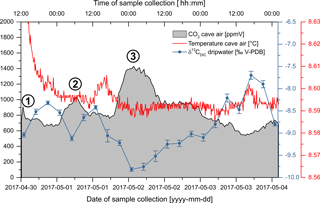
Figure 1Hardware design of the GUARD autosampler (top view, schematical). Water samples are pumped directly into vials that are permanently gastight by rubber septa. The shown set-up comprises 48 sample vials, but the autosampler can be equipped with up to 160 sample vials at the given casing dimensions.
Figure 2The GUARD automated fluid sampler in detail (a, b) and in operation (c) during a 5-day case study (Sect. 4 carried out in the “Kleine Teufelshöhle” cave in the Franconian Switzerland region, Germany. At a 4 h interval, a total of 22 dripwater samples were automatically collected for subsequent analysis of the carbon isotope values (δ13CDIC) of the dissolved inorganic carbon (DIC).
2.1 Hardware design and sampling process
The main components of the GUARD autosampler comprise an intake hose, a peristaltic pump, a mobile injection system and a vial holder (Figs. 1 and 2, Table 1, Table S5 in the Supplement). To prevent any sample alteration resulting from contamination, evaporation, condensation and/or gas exchange during sample storage, the fluid samples (12 mL) are injected into airtight/gastight vials using a peristaltic pump, at a user-defined date, time and interval.
At the beginning of the first sampling interval, 12 mL of fluid are sucked into the autosampler's tubing made of flexible and chemical-resistant FKM tubing that is hydraulically connected to the water under investigation (e.g. a lake). As the tubing is just long enough to accommodate precisely 12 mL (i.e. 1247 mm), the sample is not yet injected into the corresponding vial, but at first remains inside the tubing where it is already protected from gas exchange.
At the beginning of the subsequent sampling interval, two electric motors move the sampler's X- and Y-slides via toothed rubber belts until two separate end-switches are triggered that provide positioning calibration. Both slides are then positioned directly above the first sample vial. After a 2 s safety delay, a servo screwed to the sampler's Z-slide moves the Z-slide down, until a metal double cannula attached to the front end of the FKM tubing just barely pierces the rubber septum which keeps the vial permanently airtight/gastight. After another 2 s safety delay, the sampler's pump is reactivated and the collected water is injected into the vial through one of the cannulas (the “sample cannula”) while the subsequent sample is sucked into the sampler's tubing simultaneously. As a result of this design, the sample injection always lags the sample collection by one interval.
As the sample vials are airtight, an overpressure builds up inside the vials during sample injection. Pressure equalisation is achieved via the second of the two cannulas which are soldered to one another. To achieve the maximum sample volume (here: 12 mL) this “pressure release cannula” is located 2–3 mm above the sample cannula, and the vials are filled with an overflow of several droplets. This set-up avoids any unwanted interaction (e.g. gas or isotope exchange) between the collected fluid sample and the supernatant air/gas left inside the vial. Subsequent to a 10 s safety delay implemented to allow for complete pressure equalisation and sample injection, the Z-slide with the double cannula is moved back up to its home position and the X- and Y-slides are positioned above the next sample vial. After another 2 s safety delay, the sampler enters a hibernation mode to minimise power consumption until the hibernation is interrupted by the start of the next sampling interval. After completion of a full sampling sequence, the Z-slide moves back up to its home position, the X-slide moves to its end position and the sampler waits for input from the operator.
In the set-up shown here the collected samples have a volume of 12 mL which is sufficient for most analyses, including isotope ratio mass spectrometry (IR-MS) and inductively coupled plasma mass spectrometry (ICP-MS). The pumping process takes only about 22 s and, thus, the collected sample represents the water under investigation at a given instant (integrated over 22 s). As one entire sampling step takes only 41 s (power consumption: 2.1 mAh), the autosampler is capable of high-resolution fluid sampling with a minimum interval of 1 min. This is valuable where high-frequency variations in the composition of the sampled fluid need to be resolved, for instance, in artificial tracer tests at the onset of the tracer breakthrough where samples are commonly collected at intervals as short as 1 min (Leibundgut et al., 2009). If needed, the sample volume can be modified by changing the duration of the pumping step. For example, to obtain a 100 mL sample the pumping step would take about 3 min.
Using a 12 V battery with a capacity of 40 Ah the GUARD autosampler can operate off-grid for about 100 days without interruption at a 2-day interval (one full sampling sequence), thanks to the hibernation mode during which power consumption is reduced to 16.5 mA (we are currently working on further reducing the power consumption). On such long time spans the power consumed during the actual sampling process is practically negligible. If longer operation durations are necessary, multiple batteries can be connected in parallel to increase the total capacity. The sampler can also run on 12 V Li-ion batteries if weight is an important constraint. Additionally, nearly discharged batteries can be replaced with fully charged ones without interrupting a running sampling sequence by using an electrical bypass. Of course, implementing an appropriate rectifier, the autosampler can also run on mains power, in which case runtime limitations no longer apply.
Figure 2 shows the GUARD autosampler in detail and in operation during a 5-day case study (Sect. 4) focusing on the carbon isotope geochemistry (δ13CDIC) of dripwater originating from a stalactite in a karst cave in northern Bavaria, Germany.
2.2 Electronic and software design
Most of the autosampler's electronic components are accommodated in the control unit (Fig. 1) inside an additional casing for protection. The centrepiece of the electronic design is the Arduino® Mega 2560 board which is based on an Atmel ATmega 2560 microcontroller. This microcontroller enables the autosampler to enter the hibernation mode during which power consumption is reduced 50-fold as compared to the power consumption during slide movement. It also contains a non-volatile 4 KB EEPROM memory in which the data (time and position) of the previous injection are saved temporarily. The interrupts of the hibernation mode at the beginning of each sampling interval are triggered by a real-time clock (RTC) chip that includes a separate 3 V lithium button cell battery which ensures that the program controlling the sampler operation remains active, even if the main power supply may be interrupted. The electrical circuit diagram as well as a Bill of Materials are given in the Supplement (Fig. S1, Table S6).
The program controlling the autosampler was constructed with the Arduino open-source software (version 1.8.3). The code is written in Java and can be uploaded to the board via a USB connection. A flowchart illustrating the operation of the GUARD autosampler is shown in Fig. 3.
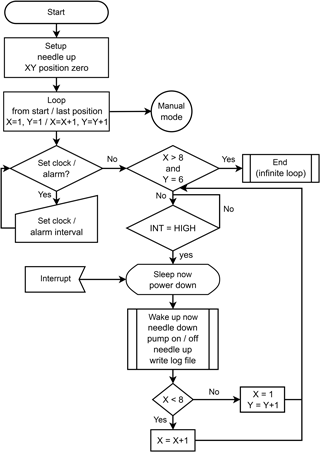
Figure 3Flowchart illustrating the autosampler's operation for a set-up comprising 48 sample vials in total, arranged in lines (x direction) of 8 and columns (y direction) of 6 vials, respectively. Once the last sample has been collected, the program enters an infinite loop and waits for input from the operator.
3.1 X–Y positioning
During and after the development of the GUARD autosampler we conducted various indoor experiments to test the mechanical functioning of our prototype. One important requirement in that respect is a precise and reliable positioning of the X- and Y-slides at the exact locations of the sample vials. High precision is especially important for an efficient use of the space available for the sample vials which are arranged as closely as possible to each other (yielding a capacity of 160 vials at the given casing dimensions). To achieve this high precision, all movements are executed by computerised numerical control (CNC). The stepper motors for the slide movement in the X and Y directions are programmed to turn in quarter-steps which correspond to a rotation of only 0.45∘. Consequently, the slide movements along the x and y axis are accurate to less than 1 mm. As the pierceable area of the rubber septa is 7 mm in diameter, there is a more than sufficient error margin of about 350 %. This prevents the double cannula from hitting the sample rack during the Z-slide's downward movement, which would cause the double cannula to deform and the respective sampling sequence to fail.
3.2 Sample injection
Another important aspect of an error-free mechanical functioning is a successful sample injection with an optimal use of the available sample volume. To demonstrate the fulfilment of this prerequisite we ran a complete sampling cycle comprising 48 tap water “samples” and analysed the size of the air bubbles remaining in the vials after sample injection. The results of this test are shown in Fig. 4: most bubbles are 9.5 mm in diameter or less, which confirms that the vials (internal diameter ≤15 mm) are filled quasi-completely during sample injection. Assuming the bubbles were a perfect sphere, they would make up ≤0.45 mL (or ≤4 % of the inner vial volume). Considering that the bubbles are in fact strongly flattened, they more likely make up ≤0.22 mL (or ≤2 % of the inner vial volume). Therefore, any sample alteration due to interactions between the fluid sample and the supernatant air/gas, for instance, due to isotopic exchange, is prevented.
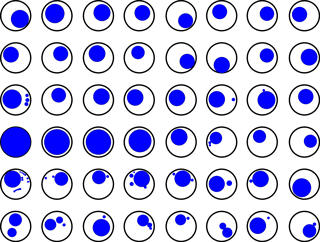
Figure 4Top view (digitised facsimile) of the sample vials (black circles) turned upside down in order to illustrate the air/gas bubbles (blue circles) remaining in the vials after sample injection.
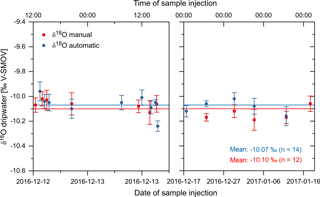
Figure 5First field testing of the GUARD autosampler: oxygen isotope values (indicated as δ18O relative to the V-SMOW international standard) in dripwater samples from a specific drip site in the “Laichinger Tiefenhöhle” karst cave in the Swabian Alb region, southern Germany. Samples were collected automatically (blue circles) over the course of 33 days (13 December 2016 to 14 January 2017) and supplemented by 12 samples collected manually (red squares) for comparison of both methods. Error bars represent measurement uncertainty. Blue and red horizontal lines indicate the overall arithmetic mean of each data set. Note the difference in scale of the x axes of the two sub-plots. Not all of the 33 samples were analysed for isotopic composition.
3.3 First field test: comparison of automatic and manual samples and long-term sample stability
With respect to the quality of the samples collected by the GUARD autosampler, there are two main prerequisites. (1) Samples need to yield identical analytical results, whether they are collected automatically or manually, and (2) samples need to be unaltered and stable, even in the long term, which is ensured by the sample vials being airtight/gastight. In order to ascertain the fulfilment of both of these criteria, we applied our device in a first field experiment in a karst cave to automatically sample the water at a specific drip site over the course of 33 days at daily intervals. For comparison, we collected 12 dripwater samples manually. We then analysed the oxygen isotopes in these water samples with CRDS on a liquid water isotope analyser (LWIA-24d; Los Gatos Research). The standards used for calibration were LGR1A, USGS 46 and USGS 48. The accuracy (<0.07 ‰) was tested by repeated measurements of the LGR 2C control standard material. The average precision of the individual measurements (n=124) was ±0.4 ‰. The isotope data are shown in Figs. 5 and 6.
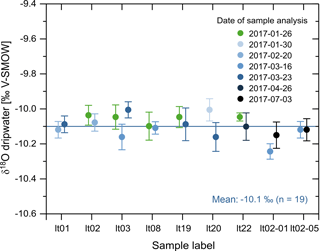
Figure 6Results of repeated δ18O measurements (circles in tones of blue) measured in the automatically collected samples together with the original δ18O data from Fig. 5 (green circles) plotted against their respective label (“lt” stands for Laichinger Tiefenhöhle). The darker the tones of blue, the later the respective measurement was repeated. For interpretation of the references to color in this figure caption, the reader is referred to the web version of this paper.
Figure 5 demonstrates that the oxygen isotope results from the automatically and manually collected samples are in good agreement with each other, especially considering the respective analytical error ranges. In two cases (13 December, around 15:00, and 22 December 2016, around 16:00), δ18O values do not seem to agree within error at first. However, as the manual samples had to be collected at least 15 min before or after the automatic collection in order to allow for sufficient sample volumes (due to the low drip rate at this specific site), this seeming mismatch can be explained by the high-frequency variability of the dripwater δ18O values at this drip site. This is best exemplified by the last two automatically collected samples in the left sub-plot in Fig. 5, where δ18O values dropped from −10.05 to −10.24 ‰ within only 30 min. The sample collected manually exactly in between these two yields an intermediate δ18O value of −10.06 ‰ and is therefore consistent with the automatically collected samples. More importantly, there is no systematic discrepancy between the automatically and manually collected samples, with the respective arithmetic mean δ18O values, calculated for the entire sampling period (both sub-plots of Fig. 5), differing by only 0.03 ‰. The results for δD are similar to the δ18O results and also confirm the long-term stability of the samples (Fig. S3).
In order to demonstrate that the sample vials are completely airtight and remain so even after the double cannula has pierced the rubber septa during sample injection, we measured the oxygen isotopic composition of nine different samples (stored in a fridge at 11.2 ∘C) repeatedly over a time interval of 6 months. The results (Fig. 6) confirm the long-term stability of the samples: if the vials were not airtight, evaporation would have led to a preferential removal of isotopically light water molecules from the water samples due to their higher vapour pressure (e.g. Hoefs, 2015) and, consequently, to an increase in the δ18O value of the remaining water sample over time. Such a positive trend is not present in the data and the results from the repeated measurements agree well with the initial ones. The difference in δ18O values between initial and repeated measurements ranges from 0.00 ‰ (lt02-05) to 0.15 ‰ (between the second and third measurements of sample lt03), but averages out at −0.01 ‰ over all measurements (median also −0.01 ‰), indicating that there is no systemic discrepancy between the initial and repeated analyses. The results for δD are similar to the δ18O results and also confirm the long-term stability of the samples (Fig. S2).
To provide the reader with a notion of the effect of evaporation on the sample δ18O values, we have calculated both evaporation and δ18O change for the conditions prevalent in our fridge (temperature 11.2 ∘C, relative humidity 24 %) and assuming an opening of the sample vial of 5 % to imitate a minor lack of airtightness. The results of these calculations (Fig. S4) demonstrate that even a small slit in a sample vial's rubber septum equalling only 5 % of the vial's inner cross section leads to a substantial shift towards higher δ18O values in the residual water over time. After 3 months (90 days), for instance, δ18O values would have risen from −10.1 ‰ by about 1.3 to −8.8 ‰. For comparison, the difference between the lowest and highest δ18O values in Fig. 6 is still below 0.3 ‰, while those data points span an even longer period of 6 months. Most importantly, there is no positive trend in the δ18O values in Fig. 6, which illustrates that the sample vials are sealed properly, even after sample injection.
The potential and usefulness of our autosampler are demonstrated in a first case study that would have been both too expensive and time-consuming to conduct without our device. The goal was (1) to prove the existence of high-frequency (daily) variability in the carbon isotope values (δ13C) of dissolved inorganic carbon (DIC) in cave dripwaters and (2) to quantify its amplitude. This variability has important implications for the reconstruction of past environmental changes from speleothem δ13C values as these are not only a function of the dripwater δ13C signal originating from the surface environment, but also of the intensity of degassing of excess CO2 from the dripwater (Fairchild et al., 2006). However, to date δ13CDIC variability has only been documented on the seasonal and annual scale (e.g. Spötl et al., 2005; Mattey et al., 2010), certainly also due to the lack of practical solutions for the high-frequency dripwater sampling in caves.
The existence of such high-frequency variability in δ13CDIC can be postulated based on the knowledge that cave air CO2 concentrations can vary both strongly and quickly (e.g. Luetscher and Ziegler, 2012) as a response to ventilation processes (e.g. Tremaine et al., 2011): in general, strong ventilation of a cave system leads to an input of low CO2 ambient air which (partly) replaces the CO2-enriched cave air. The lowered CO2 concentration causes enhanced degassing of excess CO2 from the cave dripwater, which, in turn, results in increased dripwater δ13CDIC values, as isotopically light CO2 transits preferentially from the liquid to the gas phase (Clark and Fritz, 1999).
4.1 Study area
This case study was carried out in the “Kleine Teufelshöhle” (49∘45′17 N, 11∘25′12 E) in the Franconian Switzerland region in northern Bavaria, Germany. This cave is characterized by dynamic ventilation (forced convection). The mean annual air temperature is around 8 ∘C and the air humidity is close to saturation. This site warrants conditions suitable for a demanding field test due to (1) the lack of an electric supply network and (2) the high relative humidity which poses challenges for electrical appliances in general. The GUARD autosampler was placed at a location adequate for sampling the dripwater from a specific group of stalactites, at drip site “DS4” (Fig. 2, right).
4.2 Materials and methods
Dripwater sampling was conducted automatically at 4 h intervals over a period of 5 days, yielding a total of 22 samples. The stable carbon isotopic composition of the dripwater DIC was determined at the University of Innsbruck using continuous-flow isotope ratio mass spectrometry following the method described in Spötl (2005). Calibration of the raw results vs. the V-PDB scale is achieved using in-house calcite standards (subsequent to linearity correction) that have been calibrated against NBS-18, NBS-19, CO-1 and CO-8 reference materials. The external precision calculated over 12 standards per run is typically ≤0.07 ‰ for δ13C.
Cave air CO2 concentrations were logged every 30 min with a Vaisala GM70 hand-held unit equipped with a CO2 probe optimised for the 0–2000 ppmV range (GMP222; accuracy: ±1.5 % of the calibration value plus 2 % of the measured value). Cave air temperature (T) and relative humidity (RH) were logged at 10 min intervals with a Tinytag TGP-4500 (Gemini Loggers; accuracy: ±0.5 ∘C at 8 ∘C and ±3.0 % RH at 25 ∘C), while the combined drip rate of the stalactite cluster was logged with a Stalagmate Mark 3 (TGC-0011; Driptych) and integrated over 5 min increments.
4.3 Results
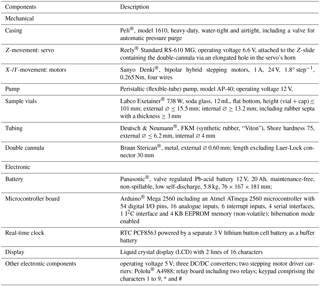
Over the duration of the 5-day case study, RH was constant at 100 % and the drip rate oscillated between 26 and 28 drops per 5 min increment (5.2 to 5.6 drops min−1). The results of the T, CO2 and δ13CDIC analyses are summarised in Fig. 7. Cave air CO2 concentrations range from 520 to 1430 ppmV, with an average of 748 ppmV (n= 476) and a median of 710 ppmV, and δ13CDIC values range from −9.8 to −7.7 ‰, with an average of −8.8 ‰ (n= 22) and a median of −8.9 ‰. CO2 concentrations peaked thrice (circled numbers in Fig. 7), with two smaller peaks of 910 and 1030 ppmV at the beginning of the monitoring period being followed by the most prominent and broad peak of 1430 ppmV that occurred during the night from 2 to 3 May 2017. All three CO2 peaks, particularly the last one, precisely coincide with troughs in the δ13CDIC values, while CO2 troughs coincide with δ13CDIC peaks, which results in a distinct negative correlation (Spearman's ) of both geochemical signals. Temperature varies only very slightly, with both average and median being 8.6 ∘C (n= 1425). Despite the low amplitude of T variations, T appears to correlate positively with δ13CDIC, but only weakly (Spearman's ρ=0.36).
4.4 Interpretations
The dripwater analyses obtained from the Kleine Teufelshöhle at 4 h resolution over 5 days clearly prove the presence of a high-frequency variability in the δ13CDIC, in addition to the already documented seasonal and interannual variability (Spötl et al., 2005; Mattey et al., 2010). In this case, the maximum amplitude is 2.1 ‰ – a change that is great enough to be resolved by state-of-the-art isotope-ratio mass spectrometers (IR-MS). While this 2.1 ‰ change occurred over a period of almost 2 days (2 May 2017 01:00 to 3 May 2017 17:00), additional variability is observed on even smaller timescales. For example, the difference in δ13CDIC values between the first local minimum (−9.04 ‰) and the first local maximum (−8.32 ‰) came about in only 8 h, with an amplitude of 0.72 ‰, suggesting rapid responses to even small changes in the ventilation regime.
The strong negative correlation between δ13CDIC values and CO2 concentrations is consistent with ventilation events that lead to decreased cave air CO2 concentrations: as high CO2 cave air is partly replaced by low CO2 ambient air degassing of excess CO2 from the dripwater is enhanced. As the process of degassing favours isotopically light CO2 molecules, the δ13CDIC values in the dripwater are increased during these ventilation events. This interpretation also seems to be confirmed by the measured T variations: although they are very small, the positive albeit moderate correlation with δ13CDIC suggests that, during ventilation, part of the cave air is replaced by relatively warm low CO2 ambient air. During winter months, it would be replaced by relatively cold but still low CO2 ambient air.
In order to characterize the changes in the dripwater δ13CDIC with respect to the cave air CO2 concentration, we have determined the amplitude of the maxima/minima in δ13CDIC and CO2 relative to their respective overall mean, simply by subtracting each maximum/minimum from the mean. This yields a total of six maximum/minimum pairs that plot very well along a linear regression line (Fig. 8). According to this regression, a change in cave air CO2 concentration of about 435 ppmV produces a change in dripwater δ13CDIC of 1 ‰ that is then transferred to the δ13C signal in the speleothem fed by this dripwater.
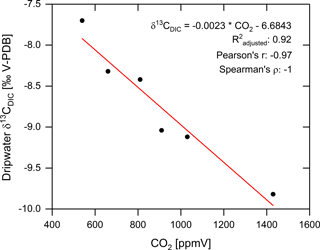
Figure 8Relationship between cave air CO2 concentrations and dripwater δ13CDIC quantified based on the six maxima/minima recorded during the case study (black circles). The relationship can be fitted very well with a linear regression (red line).
We note that potential drift effects or sample alterations that might be caused by the automatic sampling process have not yet been examined in detail. Corresponding tests using check standards of known δ13CDIC values will be performed in future studies.
With “GUARD” we have developed and tested an automated water sampler suited to field operation in remote areas and harsh conditions that injects the samples into airtight vials in order to prevent sample alteration from contamination, evaporation/condensation and/or gas exchange.
Table 2Comparison of the GUARD prototype with conventional autosamplers available on the market, represented here by the 3700C Compact from Teledyne ISCO (information retrieved from the manufacturer's website: http://www.teledyneisco.com/en-uk, last access: 30 June 2018) as well as other non-commercial autosamplers developed by members of the scientific community.
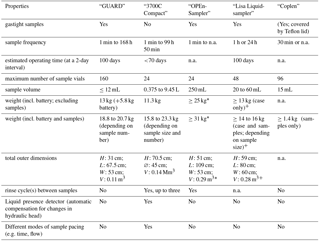
*Including the Pelican 80QT Elite Wheeled Cooler for better comparability. +Including the Zarges K470 Plus 40503 case for better comparability. n.a.: no information found.
In this paper, we have demonstrated its mechanical functioning, the long-term chemical stability of the collected samples and the potential of our autosampler in a wide variety of applications by presenting the results of a 5-day case study which would have been hardly possible without the use of our device. In this case study we have proven for the first time that cave dripwater geochemistry varies on much smaller timescales than has previously been established. Applying the GUARD autosampler we have collected enough dripwater samples at high temporal resolution to make a first contribution to quantifying the effects of ventilation events on dripwater δ13CDIC, which will help in using speleothem δ13C as a proxy for palaeoenvironmental change.
To conclude we compare our prototype with the 3700C Compact portable autosampler (Teledyne ISCO, USA) which is, to our best knowledge, the only type of device similar to our prototype already available on the market. While there are also other (bigger and heavier) models from Teledyne ISCO and similar devices offered by other companies, the 3700C Compact autosampler is representative of the technical state of the art.
The most relevant properties of both autosamplers are compared in Table 2. As might be expected when comparing a prototype with a market-ready product, it is evident that the GUARD autosampler lacks specific features that enhance the end-user comfort, such as rinse cycles between samples, an automatic compensation for changes in hydraulic head and different modes of sampling pacing. Both autosamplers are similar in weight and size. Almost a third of the GUARD's weight is due to the Pb-acid battery used in the presented set-up. The battery can be transported separately from the autosampler or can be replaced with lighter Li-ion batteries to reduce weight. Most importantly, however, the GUARD autosampler is capable of collecting gastight samples in a considerably higher maximum number of sample vials. Thanks to a wide range of selectable sample frequencies and its capacity to operate for extended periods of time, the GUARD autosampler is well suited for long-term sampling projects where a large number of samples need to be collected.
As existing autosamplers such as the 3700C Compact feature neither gastight samples nor high numbers of sample vials, the GUARD autosampler is less competitive with existing autosamplers as much as it closes a market gap in long-term monitoring where samples definitely need to be gastight with respect to air/gas exchange to prevent sample alteration. Within this sector, the GUARD autosampler offers many opportunities in various applications.
One example of such an application is the investigation of the hydrological cycle based on isotopes in all sorts of water bodies, including rainwater. As mentioned in the Introduction, for this purpose the IAEA supplies researchers with isotope data generated from monthly composite samples of rainwater collected at the ∼1000 GNIP stations worldwide. If these stations were supplemented with GUARD autosamplers, much shorter sampling intervals would become possible, which would enable researchers to investigate shorter-term variability in precipitation isotope systematics to improve our understanding of the underlying processes. To achieve this, sampling frequency needs to be at least high enough to resolve different precipitation events (“event-based” sampling). For instance, only by using such event-based data were Celle-Jeanton et al. (2001) able to demonstrate characteristic differences in the isotopic composition of rainwater in the Mediterranean coastal region of France the authors attributed to different types of synoptic weather systems. As the synoptic weather situation can change rather quickly, monthly rainwater isotope data would have most likely been of insufficient temporal resolution to identify this relationship between isotope composition and synoptics. Naturally, the increased number of samples generated by high-frequency sampling needs to be considered.
In addition, paraffin oil would not be required to prevent evaporation and increased maintenance of CRDS instruments could be avoided. The GUARD autosampler could also be applied at the ∼750 stations of the Global Network for Isotopes in Precipitation (GNIR), also coordinated by the IAEA. Especially in very remote areas, the application of GUARD samplers would be a cost-effective solution to supplement GNIP and/or GNIR stations and it might even facilitate the installation of new stations too remote for regular manual sample collection.
The GUARD autosampler could also be applied at the ∼750 stations of the IAEA's Global Network for Isotopes in Precipitation (GNIR). Especially in very remote areas, the application of GUARD samplers would be a cost-effective solution to supplement GNIP and/or GNIR stations and it might even facilitate the installation of new stations too remote for regular manual sample collection.
Due to the temporally discontinuous nature of rainfall, automatic rainwater sampling requires (1) sample pre-collection for temporary storage of rainwater until a sufficient sample volume is available while minimising or even preventing evaporation and (2) a detector such as a photo sensor to end hibernation and trigger sample collection once a sufficient sample volume has been provided by rainfall. For the case studies in karst caves presented in this paper we applied a specifically designed pre-collection container (“pre-collector”) with an internal volume of exactly 12 mL (Fig. S7). During dripwater pre-collection a 3-D-printed floating body inside the pre-collector would rise until it seals the pre-collector once it is completely filled with dripwater. Any dripwater in excess of 12 mL spills over through a small hole at the top of the pre-collector. It is important to note that, at its current set-up, the GUARD autosampler does not comprise a sample volume detector and is therefore not suited for rainwater sampling. As automatic rainwater sampling would be beneficial in numerous applications, such a detector certainly represents a useful future extension to the current GUARD system.
Other examples of a promising application are dripwater monitoring schemes in karst caves that are necessary especially, if not exclusively, in speleothem science (Ford and Williams, 2007; Fairchild and Baker, 2012). The case study presented here illustrates well the GUARD's potential for offering new research opportunities. As most cave sites are located far from researchers' offices and are often difficult to get to, there is a great need for automation in dripwater monitoring studies. The sampler's application in high-frequency (short-interval) dripwater sampling will enable researchers to identify, resolve and quantify short-term variability in dripwater geochemistry and to better understand these complex cave systems – a prerequisite for reliable reconstructions of past climates and environments from speleothem proxies.
All data used in this study are available upon request from the corresponding author. In accordance with the principle of open science, further crucial data and information that enable the construction of a GUARD autosampler are available in the KITopen repository, including the Arduino sketch in its latest version (https://doi.org/10.5445/IR/1000085057, Hartmann and Wachter, 2018), assembly instructions (https://doi.org/10.5445/IR/1000085058, Hartmann and Wachter, 2018) and 3-D models of crucial components of the sampler (https://doi.org/10.5445/IR/1000085059, Hartmann and Wachter, 2018), such as the servo connector, the double-cannula holder and the sample rack.
The supplement related to this article is available online at: https://doi.org/10.5194/hess-22-4281-2018-supplement.
AH, ML and RW conceptualised the device and the case studies. RW and AH built the device and tested it with the help of PH. ML performed the stable carbon isotope analysis. AH and PH conducted the cave monitoring (Kleine Teufelshöhle). AH prepared the manuscript with contributions from all co-authors.
The authors declare that they have no conflict of interest.
The technical developments leading to the prototype presented here were
partly conducted in the framework of the Karst Water Technologies (KaWaTech)
Project in Vietnam (CLIENTFE2-069) which was funded by the German Federal
Ministry of Education and Research (BMBF). We would like to thank the FMER
for the financial support. Furthermore, we would like to express our thanks
to the cave associations “Höhlen- und Heimatverein Laichingen e.V.” and
“Forschungsgruppe Höhle und Karst Franken e.V.” for their support and
for granting us access to the cave sites. We direct special thanks to Rolf
Riek and Dieter and Annette Preu for their help. Marc Luetscher acknowledges
the Tiroler Wissenschaftsfonds for partial funding of an early prototype
(AP715009). We would like to thank Rolf Hut and an anonymous reviewer for their
comments that helped improve the manuscript. We also thank Nils Michelsen,
Stefan Terzer-Wassmuth and Luis Araguás-Araguás for their helpful
short comments.
The article processing
charges for this open-access
publication were covered by a
Research
Centre of the Helmholtz Association.
Edited by: Markus Weiler
Reviewed by: Rolf Hut
and one anonymous referee
Ahuja, S.: Monitoring water quality. Pollution assessment, analysis, and remediation, Elsevier, Amsterdam, Boston, 2013.
Appelo, C. A. J. and Postma, D.: Geochemistry, groundwater and pollution, 2nd edn., CRC Press Taylor & Francis Group, Boca Raton, London, New York, 2005.
Celle-Jeanton, H., Travi, Y., and Blavoux, B.: Isotopic typology of the precipitation in the Western Mediterranean region at three different time scales, Geophys. Res. Lett., 28, 1215–1218, 2001.
Chapin, T. P.: High-frequency, long-duration water sampling in acid mine drainage studies. A short review of current methods and recent advances in automated water samplers, Appl. Geochem., 59, 118–124, 2015.
Clark, I. D. and Fritz, P.: Environmental isotopes in hydrogeology, 2nd print, corr., Lewis Publishers, Boca Raton, USA, 1999.
Coplen, T. B., Neiman, P. J., White, A. B., Landwehr, J. M., Ralph, M., and Dettinger, M. D.: Extreme changes in stable hydrogen isotopes and precipitation characteristics in a landfalling Pacific storm, Geophys. Res. Lett., 35, L21808, https://doi.org/10.1029/2008GL035481, 2008
Fairchild, I. J. and Baker, A.: Speleothem Science. From Process to Past Environments, 1st edn., Wiley-Blackwell, Chichester, 2012.
Fairchild, I. J., Smith, C. L., Baker, A., Fuller, L., Spötl, C., Mattey, D., McDermott, F., and E. I. M. F.: Modification and preservation of environmental signals in speleothems, Earth-Sci. Rev., 75, 105–153, 2006.
Ford, D. C. and Williams, P. W.: Karst hydrogeology and geomorphology, rev. edn., John Wiley & Sons, Chichester, England, Hoboken, New Jersey, 2007.
Hartmann, A. and Wachter, R.: GUARD autosampler – Arduino sketch, https://doi.org/10.5445/IR/1000085057, 2018.
Hartmann, A. and Wachter, R.: GUARD autosampler – Assembly instructions, https://doi.org/10.5445/IR/1000085058, 2018.
Hartmann, A. and Wachter, R.: GUARD autosampler – Print-outs, https://doi.org/10.5445/IR/1000085059, 2018.
Hiscock, K. M. and Bense, V. F.: Hydrogeology, Principles and practice, 2nd edn., Wiley, Chichester, 2014.
Hoefs, J.: Stable isotope geochemistry, 7th edn., Springer, Cham, 2015.
IAEA/WMO: Global Network for Isotopes in Precipitation (GNIP) Database, IGBP PAGES/World Data Center-A for Paleoclimatology Data Contribution Series # 94-005, NOAA/NGDC Paleoclimatology Program, Boulder CO, USA, 1994.
Leibundgut, C., Maloszewski, P., and Klls, C.: Tracers in Hydrology, John Wiley & Sons Ltd, Chichester, UK, 2009.
Luetscher, M. and Ziegler, F.: CORA – a dedicated device for carbon dioxide monitoring in cave environments, Int. J. Speleol., 41, 273–281, 2012.
Mattey, D. P., Fairchild, I. J., Atkinson, T. C., Latin, J.-P., Ainsworth, M., and Durell, R.: Seasonal microclimate control of calcite fabrics, stable isotopes and trace elements in modern speleothem from St Michaels Cave, Gibraltar, Geol. Soc. Spec. Publ., 336, 323–344, 2010.
Nelke, M., Selker, J. S., and Udell, C.: The OPEnSampler: A Low-Cost, Low-Weight, Customizable and Modular Open Source 24-Unit Automatic Water Sampler, Abstract H41J-1596 presented at 2017 Fall Meeting, AGU, New Orleans, LA, 11–15 December 2017.
Reemtsma, T. and Jekel, M. (Eds.): Organic pollutants in the water cycle, Properties, occurrence, analysis and environmental relevance of polar compounds, Wiley-VCH, Weinheim, Chichester, 2010.
Spötl, C.: A robust and fast method of sampling and analysis of δ13C of dissolved inorganic carbon in ground waters, Isot. Environ. Healt. S., 41, 217–221, 2005.
Spötl, C., Fairchild, I. J., and Tooth, A. F.: Cave air control on dripwater geochemistry, Obir Caves (Austria), Implications for speleothem deposition in dynamically ventilated caves, Geochim. Cosmochim. Ac., 69, 2451–2468, 2005.
Terzer, S., Wassenaar, L. I., Douence, C., and Araguas-Araguas, L.: An assessment of the isotopic (2H/18O) integrity of water samples collected and stored by unattended precipitation totalizers, Geophysical Research Abstracts, 18, EGU2016-15992. 2016.
Tremaine, D. M., Froelich, P. N., and Wang, Y.: Speleothem calcite farmed in situ: Modern calibration of δ18O and δ13C paleoclimate proxies in a continuously-monitored natural cave system, Geochim. Cosmochim. Ac., 75, 4929–4950, 2011.
- Abstract
- Introduction
- Methodology: autosampler set-up and design
- Demonstration of the autosampler's functioning
- Case study: high-resolution drip sampling for speleothem science
- Summary and potential applications
- Data availability
- Author contributions
- Competing interests
- Acknowledgements
- References
- Supplement
- Abstract
- Introduction
- Methodology: autosampler set-up and design
- Demonstration of the autosampler's functioning
- Case study: high-resolution drip sampling for speleothem science
- Summary and potential applications
- Data availability
- Author contributions
- Competing interests
- Acknowledgements
- References
- Supplement